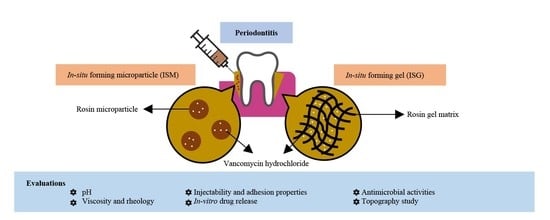
Design and Comparative Evaluation of Vancomycin HCl-Loaded Rosin-Based In Situ Forming Gel and Microparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Formulations
2.2.1. Preparation of In-Situ Forming Gel (ISG)
2.2.2. Preparation of In Situ Forming Microparticles (ISM)
2.3. Evaluation of the ISG and ISM Systems
2.3.1. Appearance and Phase Separation of ISM Emulsion
2.3.2. pH Measurement
2.3.3. Viscosity and Rheology Behavior
2.3.4. Injectability Test
2.3.5. Adhesion Properties
2.3.6. In-Vitro Gel and Microparticle Transformation
2.3.7. In-Vitro Drug Release
2.3.8. Topography
2.3.9. Antimicrobial Activities
2.4. Statistical Analysis
3. Results and Discussion
3.1. Appearance and Phase Separation
3.2. pH Measurement
3.3. Viscosity
3.4. Injectability Test
3.5. Adhesion Properties
3.6. In Vitro Transformation
3.7. Size of In Situ Forming Microparticles (ISM)
3.8. Drug Release
3.9. Surface Topography
3.10. Antimicrobial Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satturwar, P.M.; Fulzele, S.V.; Dorle, A.K. Evaluation of polymerized rosin for the formulation and development of transdermal drug delivery system: A technical note. AAPS PharmSciTech 2005, 6, E649–E654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, O.P.; Malviya, R.; Balsal, V.; Sharma, P.K. Rosin an important polymer for drug delivery: A short review. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 35–37. [Google Scholar]
- Söderberg, T.A.; Gref, R.; Holm, S.; Elmros, T.; Hallmans, G. Antibacterial activity of rosin and resin acids in vitro. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1990, 24, 199–205. [Google Scholar] [CrossRef]
- Kranz, H.; Brazeau, G.A.; Napaporn, J.; Martin, R.L.; Millard, W.; Bodmeier, R. Myotoxicity studies of injectable biodegradable in-situ forming drug delivery systems. Int. J. Pharm. 2001, 212, 11–18. [Google Scholar] [CrossRef]
- Do, M.; Neut, C.; Metz, H.; Delcourt, E.; Mäder, K.; Siepmann, J. In-situ forming composite implants for periodontitis treatment: How the formulation determines system performance. Int. J. Pharm. 2015, 486, 38–51. [Google Scholar] [CrossRef]
- Do, M.; Neut, C.; Metz, H.; Delcourt, E.; Siepmann, J.; Mäder, K. Mechanistic analysis of PLGA/HPMC-based in-situ forming implants for periodontitis treatment. Eur. J. Pharm. Biopharm. 2015, 94, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Kopytynska-Kasperczyk, A.; Dobrzynski, P.; Pastusiak, M.; Jarzabek, B.; Prochwicz, W. Local delivery system of doxycycline hyclate based on ϵ-caprolactone copolymers for periodontitis treatment. Int. J. Pharm. 2015, 491, 335–344. [Google Scholar] [CrossRef]
- Phaechamud, T.; Senarat, S.; Puyathorn, N.; Praphanwittaya, P. Solvent exchange and drug release characteristics of doxycycline hyclate-loaded bleached shellac in situ-forming gel and -microparticle. Int. J. Biol. Macromol. 2019, 135, 1261–1272. [Google Scholar] [CrossRef]
- Khaing, E.M.; Intaraphairot, T.; Chuenbarn, T.; Chantadee, T.; Phaechamud, T. Natural resin-based solvent exchange induced in-situ forming gel for vancomycin HCl delivery to periodontal pocket. Mater. Today Proc. 2021, 47, 3585–3593. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-based antisolvent-induced in situ forming matrix for crevicular pocket delivery of vancomycin hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef]
- Chantadee, T.; Sawangsri, P.; Santimaleeworagun, W.; Phaechamud, T. Vancomycin hydrochloride-loaded stearic acid/lauric acid in situ forming matrix for antimicrobial inhibition in patients with joint infection after total knee arthroplasty. Mater. Sci. Eng. C 2020, 115, 110761. [Google Scholar] [CrossRef] [PubMed]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Vancomycin HCl-loaded lauric acid in situ-forming gel with phase inversion for periodontitis treatment. J. Drug Deliv. Sci. Technol. 2020, 57, 101615. [Google Scholar] [CrossRef]
- Yapar, E.; Inal, Ö.; Ozkan, Y.; Baykara, T. Injectable in situ forming microparticles: A novel drug delivery system. Trop. J. Pharm. Res. 2012, 11, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Kranz, H.; Yilmaz, E.; Brazeau, G.A.; Bodmeier, R. In vitro and in vivo drug release from a novel in situ forming drug delivery system. Pharm. Res. 2008, 25, 1347–1354. [Google Scholar] [CrossRef]
- Voigt, M.; Koerber, M.; Bodmeier, R. Improved physical stability and injectability of non-aqueous in situ PLGA microparticle forming emulsions. Int. J. Pharm. 2012, 434, 251–256. [Google Scholar] [CrossRef]
- Collins, J.F. Effect of vancomycin on plaque after periodontal surgery. J. Dent. Res. 1970, 49, 1478–1480. [Google Scholar] [CrossRef]
- Gibson, W.A. Antibiotics and periodontal disease: A selective review of the literature. J. Am. Dent. Assoc. 1982, 104, 213–218. [Google Scholar] [CrossRef]
- Kaslick, R.S.; Tuckman, M.A.; Chasens, A.I. Effect of topical vancomycin on plaque and chronic gingival inflammation. J. Periodontol. 1973, 44, 366–368. [Google Scholar] [CrossRef]
- Phaechamud, T.; Lertsuphotvanit, N.; Praphanwittaya, P. Viscoelastic and thermal properties of doxycycline hyclate-loaded bleached shellac in situ -forming gel and–microparticle. J. Drug Deliv. Sci. Technol. 2018, 44, 448–456. [Google Scholar] [CrossRef]
- Do, M.; Neut, C.; Delcourt, E.; Certo, T.S.; Siepmann, F. In situ forming implants for periodontitis treatment with improved adhesive properties. Eur. J. Pharm. Biopharm. 2014, 88, 342–350. [Google Scholar] [CrossRef]
- Haider, M.; Elsayed, I.; Ahmed, I.; Fares, A. In Situ-forming microparticles for controlled release of rivastigmine: In vitro optimization and in vivo evaluation. Pharmaceuticals 2021, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Chanyaboonsub, N.; Setthajindalert, O. Doxycycline hyclate-loaded bleached shellac in situ forming microparticle for intraperiodontal pocket local delivery. Eur. J. Pharm. Sci. 2016, 93, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Praphanwittaya, P.; Laotaweesub, K. Solvent effect on fluid characteristics of doxycycline hyclate-loaded bleached shellac in situ-forming gel and -microparticle formulations. J. Pharm. Investig. 2017, 48, 409–419. [Google Scholar] [CrossRef]
- Rein, S.M.T.; Lwin, W.W.; Tuntarawongsa, S.; Phaechamud, T. Meloxicam-loaded solvent exchange-induced in situ forming beta-cyclodextrin gel and microparticle for periodontal pocket delivery. Mater. Sci. Eng. C 2020, 117, 111275. [Google Scholar] [CrossRef]
- Jain, N.; Lai, P.-C.; Walters, J.D. Effect of gingivitis on azithromycin concentrations in gingival crevicular fluid. J. Periodontol. 2012, 83, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- Karastogianni, S.; Girousi, S.; Sotiropoulos, S. pH: Principles and measurement. In Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2016; pp. 333–338. [Google Scholar]
- Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K.; Wierzbicka, E. Advances in rosin-based chemicals: The latest recipes, applications and future trends. Molecules 2019, 24, 1651. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M.; Das Gupta, V. Stability of Vancomycin hydrochloride solutions at various ph values as determined by high-performance liquid chromatography. Drug Dev. Ind. Pharm. 1995, 21, 257–264. [Google Scholar] [CrossRef]
- Godet, M.; Simar, J.; Closset, M.; Hecq, J.-D.; Braibant, M.; Soumoy, L.; Gillet, P.; Jamart, J.; Bihin, B.; Galanti, L. Stability of concentrated solution of vancomycin hydrochloride in syringes for intensive care units. Pharm. Technol. Hosp. Pharm. 2018, 3, 23–30. [Google Scholar] [CrossRef]
- Balesteros, M.R.; Tavares, M.F.M.; Ribeiro, S.J.L.; Polachini, F.C.; Messaddeq, Y.; de Oliveira, M.A.L. Determination of olive oil acidity by CE. Electrophoresis 2007, 28, 3731–3736. [Google Scholar] [CrossRef]
- Rungseevijitprapa, W.; Bodmeier, R. Injectability of biodegradable in situ forming microparticle systems (ISM). Eur. J. Pharm. Sci. 2009, 36, 524–531. [Google Scholar] [CrossRef]
- Nierat, T.H.; Musameh, S.M.; Abdel-Raziq, I.R. Temperature-dependent of olive oil viscosity. Mater. Sci. Ind. J. 2014, 11, 233–238. [Google Scholar]
- McPherson, J.S.; Dixon, S.A.; Townsend, R.; Vandewalle, K.S. Effect of needle design on pain from dental local anesthetic injections. Anesth. Prog. 2015, 62, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, D.; Wang, X.; Cheng, X.; Jiang, X. Friction modeling and analysis of injection process in squeeze casting. J. Mater. Process. Technol. 2017, 239, 42–51. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Sholapurkar, A.; Sharma, D.; Glass, B.; Miller, C.; Nimmo, A.; Jennings, E. Professionally delivered local antimicrobials in the treatment of patients with periodontitis—A narrative review. Dent. J. 2020, 9, 2. [Google Scholar] [CrossRef]
- Hellmann, C.; Treat, N.D.; Scaccabarozzi, A.D.; Hollis, J.R.; Fleischli, F.D.; Bannock, J.H.; de Mello, J.; Michels, J.J.; Kim, J.-S.; Stingelin, N. Solution processing of polymer semiconductor: Insulator blends-tailored optical properties through liquid-liquid phase separation control. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 304–310. [Google Scholar] [CrossRef]
- Khaing, E.M.; Mahadlek, J.; Okonogi, S.; Phaechamud, T. Lime peel oil-incorporated rosin-based antimicrobial in situ forming gel. Gels 2022, 8, 169. [Google Scholar] [CrossRef]
- Dhanraj, M.; Benita, P.; Jain, A.R.; Vamar, A. Effect of sub-gingival margins influencing periodontal health—A systematic review and meta analysis. Biomed. Pharmacol. J. 2017, 10, 739–747. [Google Scholar]
- Lizambard, M. Implants se formant in-situ pour le traitement de la parodontite. In Médecine Humaine et Pathologie; Université de Lille: Lille, France, 2019. [Google Scholar]
- Timotius, D.; Kusumastuti, Y.; Imani, N.A.C.; Rochmadi, R.; Putri, N.R.E.; Rahayu, S.S.; Wirawan, S.K.; Ikawati, M. Kinetics of drug release profile from maleic anhydride-grafted-chitosan film. Mater. Res. Express 2020, 7, 046403. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Rubini, D.; Giovagnoli, S.; Ricci, M.; Blasi, P.; Rossi, C. Novel mucoadhesive buccal formulation containing metronidazole for the treatment of periodontal disease. J. Control. Release 2004, 95, 521–533. [Google Scholar] [CrossRef]
- Luan, X.; Bodmeier, R. In situ forming microparticle system for controlled delivery of leucprolide acetate: Influence of the formulation and processing parameter. Eur. J. Pharm. Sci. 2006, 27, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Thurein, S.M.; Chantadee, T. Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel. Asian J. Pharm. Sci. 2017, 13, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Chantadee, T.; Sirirak, J.; Hoshino, T.; Phaechamud, T. Augmentative molecular aspect for phase inversion of vancomycin hydrochloride-loaded fatty acid in situ forming matrices. Mater. Des. 2021, 199, 109429. [Google Scholar] [CrossRef]
- Sojitra, C.; Tehare, A.; Dholakia, C.; Sudhakar, P.; Agarwal, S.; Singh, K.K. Development and validation of residual solvent determination by headspace gas chromatography in imatinib mesylate API. SN Appl. Sci. 2019, 1, 233. [Google Scholar] [CrossRef] [Green Version]
- Tjäderhane, L.; Mehtälä, P.; Scaffa, P.M.C.; Vidal, C.; Pääkkönen, V.; Breschi, L.; Hebling, J.; Tay, F.R.; Nascimento, F.D.; Pashley, D.H.; et al. The effect of dimethyl sulfoxide (DMSO) on dentin bonding and nanoleakage of etch-and-rinse adhesives. Dent. Mater. 2013, 29, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Nasra, M.M.A.; Khiri, H.M.; Hazzah, H.A.; Abdallah, O.Y. Formulation, in-vitro characterization and clinical evaluation of curcumin in-situ gel for treatment of periodontitis. Drug Deliv. 2017, 24, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Alhasso, B.; Ghori, M.U.; Conway, B.R. Systematic review on the effectiveness of essential and carrier oils as skin penetration enhancers in pharmaceutical formulations. Sci. Pharm. 2022, 90, 14. [Google Scholar] [CrossRef]
- Mehtälä, P.; Pashley, D.; Tjäderhane, L. Effect of dimethyl sulfoxide on dentin collagen. Dent. Mater. 2017, 33, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Mehmood, N.; Singh, U. Enhancing the resin-dentin bond with DMSO: A narrative review. Glob. J. Oral Sci. 2019, 5, 40–46. [Google Scholar] [CrossRef]
- Hebling, J.; Bianchi, L.; Basso, F.; Scheffel, D.; Soares, D.; Carrilho, M.; Pashley, D.; Tjäderhane, L.; Costa, C.A.D.S. Cytotoxicity of dimethyl sulfoxide (DMSO) in direct contact with odontoblast-like cells. Dent. Mater. 2015, 31, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, A.; Zhao, X.; Liu, X.; Wang, D.; Sun, F.; Li, Y. Design of a long-term antipsychotic in situ forming implant and its release control method and mechanism. Int. J. Pharm. 2012, 427, 284–292. [Google Scholar] [CrossRef]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Phaechamud, T. Saturated fatty acid-based in situ forming matrices for localized antimicrobial delivery. Pharmaceutics 2020, 12, 808. [Google Scholar] [CrossRef]
- National Institute of Dental and Craniofacial Research. Periodontal (Gum) Disease Cause, Symptoms and Treatments; NIH Publication: Bethesda, MD, USA, 2013. [Google Scholar]
- Awan, M.; Buriak, I.; Fleck, R.; Fuller, B.; Goltsev, A.; Kerby, J.; Lowdell, M.; Mericka, P.; Petrenko, A.; Petrenko, Y.; et al. Dimethyl sulfoxide: A central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen. Med. 2020, 15, 1463–1491. [Google Scholar] [CrossRef] [PubMed]
- De Ménorval, M.-A.; Mir, L.M.; Fernández, M.L.; Reigada, R. Effects of dimethyl sulfoxide in cholesterol-containing lipid membranes: A comparative study of experiments in silico and with cells. PLoS ONE 2012, 7, e41733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarrand, J.J.; LaSala, P.R.; Han, X.-Y.; Rolston, K.V.; Kontoyiannis, D.P. Dimethyl sulfoxide enhances effectiveness of skin antiseptics and reduces contamination rates of blood cultures. J. Clin. Microbiol. 2012, 50, 1552–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Phaechamud, T. Mixed solvent-lauric acid solvent-exchange induced in situ forming gel. Key Eng. Mater. 2019, 819, 195–201. [Google Scholar] [CrossRef]
- Senarat, S.; Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Phaechamud, T. Alpha-mangostin phase inversion induced in situ forming gel. Key Eng. Mater. 2019, 819, 202–208. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, H.; Hao, L.; Chen, H.; Zhou, X. Natural rosin modified carboxymethyl cellulose delivery system with lowered toxicity for long-term pest control. Carbohydr. Polym. 2021, 259, 117749. [Google Scholar] [CrossRef]
- Khurana, S.; Mathur, P.; Malhotra, R. Staphylococcus aureus at an Indian tertiary hospital: Antimicrobial susceptibility and minimum inhibitory concentration (MIC) creep of antimicrobial agents. J. Glob. Antimicrob. Resist. 2018, 17, 98–102. [Google Scholar] [CrossRef]
- Kshetry, A.O.; Pant, N.D.; Bhandari, R.; Khatri, S.; Shrestha, K.L.; Upadhaya, S.K.; Poudel, A.; Lekhak, B.; Raghubanshi, B.R. Minimum inhibitory concentration of vancomycin to methicillin resistant Staphylococcus aureus isolated from different clinical samples at a tertiary care hospital in Nepal. Antimicrob. Resist. Infect. Control 2016, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Citron, D.M.; Tyrrell, K.L.; Merriam, C.V.; Goldstein, E.J.C. In Vitro Activities of CB-183,315, Vancomycin, and metronidazole against 556 strains of clostridium difficile, 445 other intestinal anaerobes, and 56 enterobacteriaceae species. Antimicrob. Agents Chemother. 2012, 56, 1613–1615. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.N.; Thornsberry, C. Antimicrobial susceptibility of streptococcus mutans isolated from patients with endocarditis. Antimicrob. Agents Chemother. 1974, 5, 268–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinelli, F.; Saadati, M.; Zare, E.N.; Makvandi, P.; Masi, M.; Sacchetti, A.; Rossi, F. A perspective on the applications of functionalized nanogels: Promises and challenges. Int. Mater. Rev. 2022, 11, 1–25. [Google Scholar] [CrossRef]








| Formulation | Vancomycin HCl (V) | Rosin (R) | DMSO | Olive Oil | GMS |
|---|---|---|---|---|---|
| 20RV | 1.0 | 20 | 79.0 | - | - |
| 30RV | 1.0 | 30 | 69.0 | - | - |
| 40RV | 1.0 | 40 | 59.0 | - | - |
| 50RV | 1.0 | 50 | 49.0 | - | - |
| 60RV | 1.0 | 60 | 39.0 | - | - |
| 20RV ISM | 0.5 | 10 | 39.5 | 42.5 | 7.5 |
| 30RV ISM | 0.5 | 15 | 34.5 | 42.5 | 7.5 |
| 40RV ISM | 0.5 | 20 | 29.5 | 42.5 | 7.5 |
| 50RV ISM | 0.5 | 25 | 24.5 | 42.5 | 7.5 |
| 60RV ISM | 0.5 | 30 | 19.5 | 42.5 | 7.5 |
| V in DMSO | 1.0 | - | 99.0 | - | - |
| Formula | pH | Viscosity (cPs) | Size (µm) | |
|---|---|---|---|---|
| Emulsion | Microparticle | |||
| DMSO | 11.20 ± 0.17 | 3.53 ± 0.08 | - | - |
| 20RV | 5.89 ± 0.05 | 7.49 ± 0.60 | - | - |
| 30RV | 5.56 ± 0.06 | 16.57 ± 0.84 | - | - |
| 40RV | 5.17 ± 0.09 | 30.64 ± 1.02 | - | - |
| 50RV | 5.15 ± 0.08 | 59.98 ± 1.77 | - | - |
| 60RV | 5.02 ± 0.14 | 112.45 ± 0.98 | - | - |
| 40RV ISM | 6.48 ± 0.06 | 38.62 ± 1.33 | 98.48 ± 16.11 * | 78.63 ± 12.97 * |
| 50RV ISM | 6.46 ± 0.01 | 61.11 ± 1.67 | 125.55 ± 4.75 * | 93.81 ± 10.53 * |
| 60RV ISM | 5.93 ± 0.02 | 108.35 ± 2.45 | 137.80 ± 16.84 * | 118.32 ± 15.61 * |
| Formula | Injectability Force (N) | Injectability AUC (N.s) | Adhesion Properties | |||
|---|---|---|---|---|---|---|
| Maximum Force (N) | Adhesion Force (N) | Remaining Force (N) | Elastic Properties | |||
| 20RV | 1.10 ± 0.01 | 20.59 ± 0.42 | 3.786 ± 0.172 | −0.026 ± 0.010 | 1.364 ± 0.244 | 0.36 |
| 30RV | 2.73 ± 0.12 | 37.89 ± 2.24 | 3.579 ± 1.170 | −0.002 ± 0.001 | 2.123 ± 0.773 | 0.59 |
| 40RV | 3.10 ± 0.04 | 56.19 ± 0.57 | 0.856 ± 0.065 * | −0.050 ± 0.001 * | 0.057 ± 0.026 | 0.07 |
| 50RV | 7.98 ± 0.17 | 140.48 ± 5.29 | 0.737 ± 0.084 * | −0.051 ± 0.001 * | 0.052 ± 0.003 | 0.07 |
| 60RV | - | - | 0.420 ± 0.043 * | −0.053 ± 0.002 * | 0.026 ± 0.005 | 0.06 |
| 40RV ISM | 5.36 ± 0.02 | 92.24 ± 2.58 | 1.227 ± 0.329 * | −3.4658 ± 0.517 * | 1.128 ± 0.350 | 0.91 |
| 50RV ISM | 8.31 ± 0.73 | 135.32 ± 3.75 | 0.133 ± 0.077 * | −2.010 ± 0.525 * | 0.003 ± 0.002 | 0.03 |
| 60RV ISM | 16.83 ± 1.14 | 272.89 ± 17.69 | 0.060 ± 0.016 * | −0.192 ± 0.019 * | 0.005 ± 0.001 | 0.08 |
| Formula (% w/w) | Zero Order | First Order | Higuchi’s | POWER LAW | Release Mechanism | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| cd | msc | cd | msc | cd | msc | cd | msc | N ± S.D. | ||
| V in DMSO | 0.7061 | 0.9135 | 0.9850 | 3.9777 | 0.8905 | 1.9892 | 0.9089 | 2.0846 | 0.4035 ± 0.075 | Fickian |
| 20RV | 0.8602 | 1.7970 | 0.9899 | 4.4740 | 0.9437 | 2.7598 | 0.9495 | 2.8144 | 0.5763 ± 0.075 | Non-Fickian |
| 30RV | 0.8991 | 2.1226 | 0.9947 | 5.1257 | 0.9615 | 3.1398 | 0.9727 | 3.4319 | 0.5947 ± 0.075 | Non-Fickian |
| 40RV | 0.8960 | 2.0923 | 0.9816 | 3.8771 | 0.9615 | 3.1384 | 0.9714 | 3.3842 | 0.5894 ± 0.075 | Non-Fickian |
| 50RV | 0.8860 | 2.0004 | 0.9235 | 2.4528 | 0.9772 | 3.6630 | 0.9787 | 3.6774 | 0.5408 ± 0.075 | Non-Fickian |
| 60RV | 0.8710 | 1.8772 | 0.8678 | 1.9058 | 0.9749 | 3.5668 | 0.9742 | 3.4882 | 0.5231 ± 0.075 | Non-Fickian |
| 40RV ISM | 0.8083 | 1.4811 | 0.8660 | 1.8920 | 0.9572 | 3.0330 | 0.9556 | 2.9443 | 0.4762 ± 0.075 | Fickian |
| 50RV ISM | 0.7828 | 1.3560 | 0.7715 | 1.3585 | 0.9484 | 2.8475 | 0.9539 | 2.9061 | 0.4415 ± 0.075 | Fickian |
| 60RV ISM | 0.7383 | 1.1698 | 0.6725 | 0.9987 | 0.9245 | 2.4661 | 0.9413 | 2.6643 | 0.4131 ± 0.075 | Fickian |
| Formula | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| S. aureus | S. mutans | P. gingivalis | E. coli | |
| NSS | - | - | - | - |
| DMSO | 12.67 ± 0.58 | 11.00 ± 0.00 a | 13.67 ± 1.15 d | 12.00 ± 0.00 |
| VD | 23.67 ± 0.58 | 22.67 ± 0.58 a,b,c | 23.00 ± 0.00 d,e,f | 13.67 ± 0.58 |
| 20RV | 23.33 ± 0.58 | 22.00 ± 0.00 b | 18.33 ± 0.58 e | 12.33 ± 0.58 |
| 30RV | 22.67 ± 1.15 | 18.67 ± 0.58 b | 18.00 ± 0.00 e | 11.33 ± 0.58 |
| 40RV | 14.67 ± 0.58 | 14.33 ± 0.58 b | 14.67 ± 0.58 e | - |
| 50RV | 11.67 ± 0.58 | 11.67 ± 0.58 b | 12.67 ± 0.58 e | - |
| 60RV | 11.00 ± 0.00 | 11.00 ± 0.00 b | 11.67 ± 0.58 e | - |
| 40RV ISM | 10.00 ± 0.00 | 15.00 ± 0.00 c | 13.67 ± 0.58 f | - |
| 50RV ISM | - | 13.67 ± 0.58 c | 12.00 ± 0.00 f | - |
| 60RV ISM | - | 12.67 ± 0.58 c | 11.67 ± 0.58 f | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuenbarn, T.; Sirirak, J.; Tuntarawongsa, S.; Okonogi, S.; Phaechamud, T. Design and Comparative Evaluation of Vancomycin HCl-Loaded Rosin-Based In Situ Forming Gel and Microparticles. Gels 2022, 8, 231. https://doi.org/10.3390/gels8040231
Chuenbarn T, Sirirak J, Tuntarawongsa S, Okonogi S, Phaechamud T. Design and Comparative Evaluation of Vancomycin HCl-Loaded Rosin-Based In Situ Forming Gel and Microparticles. Gels. 2022; 8(4):231. https://doi.org/10.3390/gels8040231
Chicago/Turabian StyleChuenbarn, Tiraniti, Jitnapa Sirirak, Sarun Tuntarawongsa, Siriporn Okonogi, and Thawatchai Phaechamud. 2022. "Design and Comparative Evaluation of Vancomycin HCl-Loaded Rosin-Based In Situ Forming Gel and Microparticles" Gels 8, no. 4: 231. https://doi.org/10.3390/gels8040231