1. Introduction
For years researchers have been trying to develop target-based drug delivery systems (DDS) owing to their propensity to alleviate adverse effects and optimize therapeutic efficacy. Novel sustained release drug delivery systems are designed to control the drug concentration at the target site, reduce toxicity, optimize efficacy and provide higher patient compliance. A number of dosage forms have been developed for controlled drug delivery, such as liposomes, nano-technology, polymeric micelles, polymer-drug conjugates, dendrimers, films and hydrogels [
1,
2].
Hydrogels are considered as swellable systems which are most appropriate for site-specific sustained delivery of several drugs. They are versatile, three dimensional, crosslinked polymeric networks fabricated through solution polymerization/cross linking, bulk polymerization, free-radical polymerization, suspension or inverse-suspension polymerization and polymerization by irradiation techniques [
3,
4]. Chemically crosslinked hydrogels exhibit pronounced swelling when compared to radiation induced crosslinked hydrogels. Their potential to respond to environmental stimuli, such as pH, temperature, electric field and ionic species, has grabbed the attention of researchers since the last decade. In pH sensitive hydrogels, presence of hydrophilic groups, such as –OH, –COOH, –SO
3H, –CONH
2 and –CONH, impart swelling and deswelling character in response to pH stimulus [
5]. Exposure to media of specific pH induces generation of ionized groups which promotes volume transitions in terms of swelling due to repulsion between ionized groups [
6,
7].
Cytarabine (cytosine arabinoside or 1-β, D-arabino-furanosyl cytosine) was chosen as a typical anticancer drug. It is employed in treatment of different cancers, such as myeloid leukemia, colorectal cancer and non-Hodgkin’s lymphoma. It belongs to BCS class III. It has a short plasma half-life of 10–20 min [
8], and low oral bioavailability of about 20% [
9]. The standard dose of cytarabine used to treat acute leukemia is 3 g/m
2 through I/V route every three hours. Being water soluble, cytarabine undergoes a rapid metabolism by cytidine de-aminase to an inactive metabolite, 1-β, D-arabino-furanosyl uracil. This oxidative reaction reduces the activity of cytarabine and the resultant metabolite is eliminated rapidly through urine as uracil arabinoside [
10].
HPβCD is a hydrophilic and safe polymer with low renal toxicity. It improves functionality and solubility of drugs having low permeability (e.g., cytarabine) owing to its lipophilic inner core. It acts as a penetration enhancer and solubility modifier for lipophilic drugs [
11,
12]. In literature, it is evident that introduction of HPβCD to numerous drug delivery systems, such as buccal, transdermal and ocular, promotes solubility and bioavailability of incorporated less polar dugs [
13,
14]. HPβCD is a derivative of β-cyclodextrin (βCD) obtained by structural modifications of βCD by substituting hydroxyl (-OH) functional groups at positions C
6, C
2 or C
3. HPβCD has also been used for oral controlled delivery of different therapeutic agents. Methacrylic acid (MAA) being a monomer is pH sensitive and exhibits extraordinary swelling properties in basic environments. Methylene bis-acrylamide (MBA) is a cross linker and it possesses excellent gelation properties through physical interactions [
15,
16,
17]. Ammonium per sulphate (APS) is an initiator that is used to generate active sites at the polymer backbone and hence initiates polymerization reaction.
The aim of this study was to develop hydroxy propyl β-cyclodextrin based polymeric hydrogels with a focus on improving bioavailability of cytarabine through the oral route, thereby prolonging its half-life, reducing its dosing frequency and hence improving patients’ compliance. Moreover, the study also aimed to evaluate the effect of formulation contents on different parameters of developed hydrogels, such as swelling studies, gel fraction, release and pharmacokinetic profile of cytarabine.
4. Material and Methods
4.1. Materials
Hydroxy propyl β-cyclodextrin (99.9%), methacrylic acid (99.88%), ammonium per sulfate (99.97%), N, N-methylene bisacrylamide (MBA) (99.8%), n-hexane (99.81%) and ethanol (99.84%) were purchased from Sigma-Aldrich Co., St Louis, MO, USA. Buffering agents were obtained from Merck Germany. All the chemicals consumed in the current study were of analytical grade.
4.2. Development of HPβCD-Grafted-Poly (MAA) Polymeric Networks
Aqueous polymerization involving free radical exchange was used to develop various hydrogel preparations. A weighed amount of HPβCD (
Table 6) was dissolved in 25 mL freshly prepared distilled water with constant stirring at room temperature on a hot plate. A pre-weighed quantity of ammonium per-sulfate (APS) was dissolved in a specific quantity of water and poured dropwise into the HPβCD solution while on the hot plate stirrer. An amount (
Table 1) of methacrylic acid (MAA) was added drop wise to the above mixture. Finally, an aqueous solution of MBA was transferred slowly to the polymer-monomer mixture. The resultant solution was sonicated for at least 3–5 min to remove entrapped air. The clear solution was transferred to test tubes which were closed with aluminum paper. Test tubes were vertically racked on a steel test tube stand and the entire setup was translocated into a water bath. Initially the temperature was kept at 45 °C for 2 h followed by incremental rises in temperature, i.e., 50 °C for 2 h and finally 60 °C for 12 h. Afterwards, test tubes were allowed to cool and newly formed rod like hydrogels were removed with the help of a spatula from the test tubes. The developed hydrogels were sliced into small discs of almost 9 mm length by using a sharp-edged blade. Hydrogel discs were dipped in equimolar contents of water and alcohol (50:50) in order to remove non-reactive agents. After thorough washing, drying of discs was performed in a dry heat oven at 40 °C. After complete drying, dried hydrogel discs were stored in an airtight polyethylene plastic bag for further use [
34]. The proposed schematic presentation of HPβCD-grafted-poly (MAA) hydrogels is shown in
Figure 14.
4.3. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR scans of pure cytarabine, HPβCD, cytarabine-loaded and unloaded hydrogels were recorded to confirm structural integrity and to check for any new complex formation using an FTIR (Perkin Elmer Instrument) spectrophotometer. Scanning limit was 700–4000 cm−1.
4.4. Thermo-Analytical Investigation
In order to evaluate thermal stability and to determine phase transition temperatures, thermal studies (DSC and TGA) of polymer, pure cytarabine and cytarabine-loaded hydrogels were carried out using a thermo gravimetric analyzer (West Sussex, UK of Q5000 series) under nitrogen stream. A weighed sample loaded in a platinum pan was heated over a temperature range of 0–600 °C [
35].
4.5. X-ray Diffraction Studies
PXRD studies were done in order to verify the nature of ingredients and cytarabine within the developed hydrogels. PXRD diffractograms of pure cytarabine, HPβCD with blank and cytarabine-loaded hydrogel were recorded using an X-ray analytical Xpert powder diffractometer in the scanning range of 2
θ = 10–100° using 3°/min scanning speed [
36].
4.6. EDX Studies
EDX study was carried out to further confirm loading of cytarabine into the developed network by recording and comparing EDX scans of pure cytarabine, blank hydrogels and cytarabine containing hydrogels through scanning electron microscopy equipped with EDX—an Oxford instrument (UK).
4.7. Scanning Electron Microscopy (SEM)
The surface morphology of prepared hydrogel was studied by SEM using an optical microscope (Vega 3, Tuscan). An optimum sized sample was placed on an aluminum stump coated with a thin layer of gold (~3000 Å) and photomicrographs were collected [
37].
4.8. Sol-Gel Fraction
Dried hydrogels (1.5 g) were broken down into smaller particles and extraction of water-soluble constituents was performed in boiling water using a Soxhlet apparatus. After a period of 4 h, macro-sized particles were collected on a screen and subjected to drying in a hot air oven.
Gel fraction was measured using the following expression:
where,
Wo = weight before extraction and
Wi = weight after extraction process [
38,
39]. Measurements were conducted in triplicate.
4.9. Swelling of Hydrogels
The dried hydrogel disc (1 g) was placed in a specific volume of phosphate buffer at room temperature and taken out at predetermined time intervals of 1, 2, 3, 6, 8, 10, 12, 14, 16, 20 and 24 h in order to calculate the equilibrium swelling ratio. Excess surface water was removed using filter paper and hydrogel was weighed again. Measurements were conducted in triplicate.
Equilibrium swelling ratio was determined through using the following expression:
where,
Wf = weight of swollen hydrogel at specific time points and
Wi is the weight at dried state [
40].
4.10. Cytarabine Loading (%)
An accurately weighed amount (1.67 g) of dried hydrogel discs was soaked individually in a 1% (
w/v) phosphate buffer (pH 7.4) solution of cytarabine until there was no weight change. Swollen hydrogel discs were removed from the solution and rinsed with distilled water to get rid of surface adhered drug. Discs were placed in dry heat oven (Memmert, Schwabach, Germany) at 35 °C for complete drying [
41].
4.11. Mechanical Strength
A smooth freshly prepared hydrogel was cut and fixed in between mobile and static jaws of the tensile tester equipped with 10 kN load and TIRA software at a speed of 50 mm/min in a controlled environment. Young’s modulus was calculated from the slope of a curve drawn between tensile stress–strain curves. Similarly, stress (
σ), i.e., force per unit area and strain (
ε), i.e., changes in length were measured using the following expressions:
where,
F = actual load applied on the hydrogel sample and
r = radius of the hydrogel and
where,
lf = final length and
li = initial length [
42].
4.12. In Vitro Drug Release Studies and Kinetic Modeling
The pH-dependent release of cytarabine from HPβCD-co-poly (methacrylic acid) hydrogels (1.75 g, equivalent to 500 mg of pure cytarabine) was determined using USP Type II dissolution apparatus. Dissolution media used were 0.1 N HCl (pH 1.2) and phosphate buffer pH 7.4. Each basket was filled with 900 mL of buffer solution. The paddle speed was 50 rpm at temperature of 37 ± 0.5 °C and rotated at 50 rpm. Liquid aliquots (5 mL) were withdrawn at predetermined time intervals up to 2 h and 24 h from the acidic buffer and basic phosphate buffer pH 7.4, respectively. Fresh volume of media was replaced after each withdrawal to maintain sink conditions. Cytarabine content from the developed hydrogel was determined at 281 nm by UV spectrophotometer (UV–1600, Shimadzu, Germany) after filtering through filter paper (0.45 mm, Sartorius) and subsequent filtration. Measurements were conducted in triplicate. Drug release data was processed through zero, first order, Korsemeyer–Peppas, Higuchi and Hixson–Crowell models to explore a suitable kinetic model and to confirm the pattern of cytarabine from the developed network [
43,
44,
45].
4.13. Acute Oral Toxicity Studies
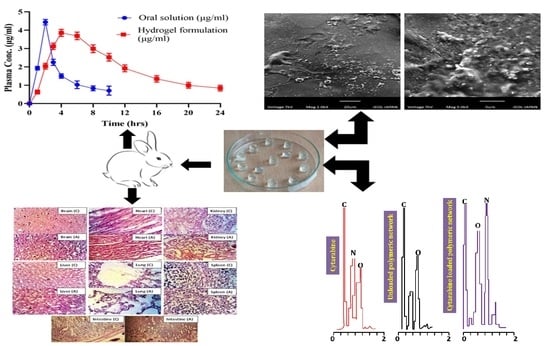
Healthy male albino rabbits (n = 18) having weight ranging from 2.3 to 2.5 kg were used in toxicity experiments. All the procedures adapted for the current study were dually evaluated and approved by the ethics committee of the faculty under its notification No. IREC2020-29.
Rabbits were kept in bird cages for 7 days with day and night 12 h cycle to acclimatize them with conditions. Rabbits were divided into three groups (n = 6), i.e., control (group A), treated (group B) and treated (group C). Rabbits in group B and group C were administered with the developed hydrogel (HPM-3), i.e., 2 and 4 g/kg, respectively, and were observed for two weeks for topical disorders, variation in body mass, consumption of water and food, physical changes, blood chemistry, RFTs, LFTs, AST and ALT levels. Rabbits were restricted from food for up to 10 h before the start of the experiment with free access to water.
At the end of the 14th day, test animals were reweighed and administered anesthesia (1 mL/kg) containing a mixture of ketamine and xylazine (70:30). Hematological samples were withdrawn from the jugular vein and transferred immediately into EDTA tubes. Rabbits were sacrificed and vital organs were removed for histopathological examination, properly washed and transferred to a container having 10% formalin as a preservative solution [
36].
4.14. In Vivo Pharmacokinetic Evaluation
An in vivo study was performed to compare the pharmacokinetic profile of the prepared cytarabine-loaded hydrogel to that of pure cytarabine using rabbits. For preclinical assessment, the rabbit model was chosen because of its easy handling, more clinically responsive behavior and ease of dose administration. Moreover, multiple samples per rabbit can be withdrawn without any harm to the animal. Healthy male albino rabbits
(n = 18) having weights ranging from 2 to 2.5 kg were procured from the animal house of the Faculty of Pharmacy and utilized in pharmacokinetic experiments. Eighteen healthy albino male rabbits (2–2.5 kg), divided into three groups (
n = 6) were used for pharmacokinetic evaluation. A cross-over study design was selected. All the rabbits were deprived of food for at least 12 h prior to dosing while giving free access to water [
17]. Pure cytarabine and cytarabine-containing hydrogels (10 mg/kg) were administered orally enclosed in hard gelatin capsules to group A and B rabbits, respectively. Nothing was administered to group C. To quantify cytarabine within plasma, a 3 mL blood sample was collected from the ear vein of rabbits at predefined time points (0, 0.5, 1, 1.5, 2.0, 2.5, 3.0, 4.0, 5.0, 6.0, 9.0, 12.0, 15.0, 18.0, 21.0, 24.0 h), transferred to EDTA tubes and centrifuged at 5000 rpm. Extracted plasma was transferred to Eppendorf tubes and stored at −20 °C in an ultra-low freezer (Sanyo-Tokyo-Japan, −80 °C).
4.15. HPLC Estimation of Cytarabine in Plasma
Frozen plasma samples were kept at normal room conditions. To 1 mL of plasma sample, 1 mL of acetonitrile was added to precipitate plasma proteins. The resultant solution was vortexed for 3–5 min and centrifuged at 5000 rpm for 10 min. Supernatant was carefully separated, filtered and diluted with mobile phase. Filtrate (approx. 20 μL) was inserted into injection port of HPLC. Mobile phase, water and acetonitrile (50:50) was operated at a flow speed of 1.2 mL/min. The total run time was 10 min and chromatograms were recorded at 272 nm [
46].
4.16. In Vitro Degradation Studies
In vitro degradation studies were conducted by the same method used by Gao et al. (2013) with slight modifications. Three liters of HCl buffer (pH 1.2) and phosphate buffer (pH 7.4) were separately prepared as per USP monograph. A measured quantity of pepsin (2.88 g/900 mL) and pancreatin (9 g/900 mL) was incorporated into the HCL buffer and phosphate buffer, respectively, to make artificial gastric fluid (AGF) and artificial intestinal fluid (AIF). For each formulation, 900 mL media was poured into a vessel of dissolution apparatus (USP Type II). Hydrogel discs of known weight were poured into these media separately. Temperature was kept at 37 °C and paddle speed was kept at 50 rpm. Hydrogel discs were removed at predetermined time intervals, i.e., 0, 0.5, 1, 1.5, 2, 3, 4, 6 and 8 h, blotted with absorbant tissue and weight measurements were conducted in order to detect weight lost at each time point [
47].