Gelling Power Alteration on Kappa-Carrageenan Dispersion through Esterification Method with Different Fatty Acid Saturation
Abstract
:1. Introduction
2. Results and Discussion
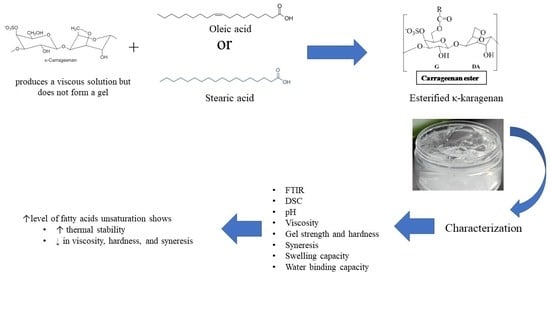
2.1. Fabrication of Ester ĸ-Carrageenan
2.2. Characterization of Physicochemical Properties
2.2.1. FTIR and TGA Analysis
2.2.2. Physical Properties of κ-Carrageenan and Its Ester Derivatives
2.2.3. Physical Stability and Properties of Optimized Gel Base
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Ester ĸ-Carrageenan
4.2. Characterization of Physicochemical Properties
4.2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
4.2.2. Thermogravimetric Analysis (TGA)
4.2.3. Organoleptic Assessment
4.2.4. pH
4.2.5. Viscosity
4.2.6. Rheological Properties
4.2.7. Gel Strength and Hardness
4.2.8. Swelling Ratio
4.2.9. Water-Binding Capacity
4.2.10. Gel Syneresis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagal-Kestwal, D.R.; Pan, M.H.; Chiang, B.H. Properties and Applications of Gelatin, Pectin, and Carrageenan Gels. In Bio Monomers for Green Polymeric Composite Materials; Wiley: Hoboken, NJ, USA, 2019; pp. 117–140. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular structure and properties of κ-carrageenan-gelatin gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Fontes-Candia, C.; Ström, A.; Lopez-Sanchez, P.; López-Rubio, A.; Martínez-Sanz, M. Rheological and structural characterization of carrageenan emulsion gels. Algal Res. 2020, 47, 101873. [Google Scholar] [CrossRef]
- Amin, P.; Riyadi, P.H.; Kurniasih, R.A.; Husni, A. Utilization of κ-carrageenan as stabilizer and thickener of honey pineapple (Ananas comosus [L. Merr]) jam. Food Res. 2022, 6, 93–98. [Google Scholar] [CrossRef]
- Skryplonek, K.; Henriques, M.; Gomes, D.; Viegas, J.; Fonseca, C.; Pereira, C.; Dmytrów, I.; Mituniewicz-Małek, A. Characteristics of lactose-free frozen yogurt with κ-carrageenan and corn starch as stabilizers. J. Dairy Sci. 2019, 102, 7838–7848. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.R.; Kuo, M.I. Effects of different carrageenan types on the rheological and water-holding properties of tofu. LWT—Food Sci. Technol. 2017, 78, 122–128. [Google Scholar] [CrossRef]
- Yuguchi, Y.; Urakawa, H.; Kajiwara, K. Structural characteristics of carrageenan gels: Various types of counter ions. Food Hydrocoll. 2003, 17, 481–485. [Google Scholar] [CrossRef]
- Langendorff, V.; Cuvelier, G.; Michon, C.; Launay, B.; Parker, A.; De Kruif, C.G. Effects of carrageenan type on the behaviour of carrageenan/milk mixtures. Food Hydrocoll. 2000, 14, 273–280. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, C.; Cui, B.; Liu, Y. Influence of cations on texture, compressive elastic modulus, sol-gel transition and freeze-thaw properties of kappa-carrageenan gel. Carbohydr. Polym. 2018, 202, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Forget, A.; Christensen, J.; Lüdeke, S.; Kohler, E.; Tobias, S.; Matloubi, M.; Thomann, R.; Prasad, V.S. Polysaccharide hydrogels with tunable stiffness and provasculogenic properties via α-helix to β-sheet switch in secondary structure. Proc. Natl. Acad. Sci. USA 2013, 110, 12887–12892. [Google Scholar] [CrossRef]
- Fomby, P.; Cherlin, A.J.; Hadjizadeh, A.; Doillon, C.J.; Sueblinvong, V.; Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; et al. Stem cells and cell therapies in lung biology and diseases: Conference report. Ann. Am. Thorac. Soc. 2010, 12, 181–204. [Google Scholar] [CrossRef]
- Mangione, M.R.; Giacomazza, D.; Bulone, D.; Martorana, V.; San Biagio, P.L. Thermoreversible gelation of κ-Carrageenan: Relation between conformational transition and aggregation. Biophys. Chem. 2003, 104, 95–105. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, B.; Qiao, D.; Yan, X.; Zhao, S.; Jia, C.; Niu, M.; Xu, Y. Addition of κ-carrageenan increases the strength and chewiness of gelatin-based composite gel. Food Hydrocoll. 2022, 128, 107565. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, S.; Du, L.; Liu, Y.; Meng, Z. Soft κ-carrageenan microgels stabilized pickering emulsion gels: Compact interfacial layer construction and particle-dominated emulsion gelation. J. Colloid Interface Sci. 2021, 602, 822–833. [Google Scholar] [CrossRef]
- Çakir, E.; Daubert, C.R.; Drake, M.A.; Vinyard, C.J.; Essick, G.; Foegeding, E.A. The effect of microstructure on the sensory perception and textural characteristics of whey protein/κ-carrageenan mixed gels. Food Hydrocoll. 2012, 26, 33–43. [Google Scholar] [CrossRef]
- Boukhatem, A.; Bouarab, K.; Yahia, A. Kappa (κ)-carrageenan as a novel viscosity-modifying admixture for cement-based materials—Effect on rheology, stability, and strength development. Cem. Concr. Compos. 2021, 124, 104221. [Google Scholar] [CrossRef]
- Hermansson, A.M.; Eriksson, E.; Jordansson, E. Effects of potassium, sodium and calcium on the microstructure and rheological behaviour of kappa-carrageenan gels. Carbohydr. Polym. 1991, 16, 297–320. [Google Scholar] [CrossRef]
- Chen, Y.; Liao, M.L.; Dunstan, D.E. The rheology of K+-κ-carrageenan as a weak gel. Carbohydr. Polym. 2002, 50, 109–116. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Yang, H. Effects of sucrose addition on the rheology and microstructure of κ-carrageenan gel. Food Hydrocoll. 2018, 75, 164–173. [Google Scholar] [CrossRef]
- Ahmad Kamil Mahmood, W.; Mizanur Rahman Khan, M.; Cheng Yee, T. Effects of Reaction Temperature on the Synthesis and Thermal Properties of Carrageenan Ester. J. Phys. Sci. 2014, 25, 123–138. [Google Scholar]
- Tosh, B.; Saikia, C.N.; Dass, N.N. Homogeneous esterification of cellulose in the lithium chloride-N,N-dimethylacetamide solvent system: Effect of temperature and catalyst. Carbohydr. Res. 2000, 327, 345–352. [Google Scholar] [CrossRef]
- Yamada, K.; Liu, J.; Kunishima, M. Development of triazine-based esterifying reagents containing pyridines as a nucleophilic catalyst. Org. Biomol. Chem. 2018, 16, 6569–6575. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Zhao, Y.; Wang, S.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Turning the morphology and wetting ability of self-assembled hierarchical structures from lignin stearoyl esters. Ind. Crops Prod. 2022, 183, 114969. [Google Scholar] [CrossRef]
- Shahbazi, M.; Rajabzadeh, G.; Ettelaie, R.; Rafe, A. Kinetic study of κ-carrageenan degradation and its impact on mechanical and structural properties of chitosan/κ-carrageenan film. Carbohydr. Polym. 2016, 142, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Rostamnia, S.; Doustkhah, E.; Baghban, A.; Zeynizadeh, B. Seaweed-derived κ-carrageenan: Modified κ-carrageenan as a recyclable green catalyst in the multicomponent synthesis of aminophosphonates and polyhydroquinolines. J. Appl. Polym. Sci. 2016, 133, 43190. [Google Scholar] [CrossRef]
- Barzetti, T.; Selli, E.; Moscotti, D.; Forni, L. Pyridine and ammonia as probes for FTIR analysis of solid acid catalysts. J. Chem. Soc. Faraday Trans. 1996, 92, 1401–1407. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, F.Q.; Chen, S.; Xiao, Q.; Weng, H.F.; Yang, Q.M.; Xiao, A.F. Preparation and characterization of κ-carrageenan modified with maleic anhydride and its application in films. Mar. Drugs 2021, 19, 486. [Google Scholar] [CrossRef]
- Koziara, B.T.; Kappert, E.J.; Ogieglo, W.; Nijmeijer, K.; Hempenius, M.A.; Benes, N.E. Thermal Stability of Sulfonated Poly(Ether Ether Ketone) Films: On the Role of Protodesulfonation. Macromol. Mater. Eng. 2016, 301, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yan, G.; Wang, J.; Kong, W.; Chang, X.; Zhuang, Y.; Meng, F. Effect of a temperature threshold on the electrorheological performance of ionic liquid crystal polyanilines. J. Mol. Liq. 2021, 326, 115299. [Google Scholar] [CrossRef]
- Heriyanto, H.; Kustiningsih, I.; Sari, D.K. The effect of temperature and time of extraction on the quality of Semi Refined Carrageenan (SRC). MATEC Web Conf. 2018, 154, 01034. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.H.; Lee, S. Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem. 2010, 118, 398–402. [Google Scholar] [CrossRef]
- Maksimov, G.V.; Volkov, V.V.; Parshina, E.Y.; Akhalaia, M.I.; Kozlova, O.V.; Derinskaya, E.V.; Revin, V.V.; Rubin, A.B. Investigation of carotenoid conformations in myelin nerve upon changes in oxygen content. Dokl. Biochem. Biophys. 2007, 417, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Rey, D.; Labuza, T. Characterization of the Effect of Solutes on the Water-Binding and Gel Strength Properties of Carrageenan. J. Food Sci. 1981, 46, 786–789. [Google Scholar] [CrossRef]
- Anderson, N.S.; Campbell, J.W.; Harding, M.M.; Rees, D.A.; Samuel, J.W.B. X-ray diffraction studies of polysaccharide sulphates: Double helix models for κ- and ι-carrageenans. J. Mol. Biol. 1969, 45, 86–99. [Google Scholar] [CrossRef]
- Millane, R.P.; Chandrasekaran, R.; Arnott, S.; Dea, I.C.M. The molecular structure of kappa-carrageenan and comparison with iota-carrageenan. Carbohydr. Res. 1988, 182, 1–17. [Google Scholar] [CrossRef]
- Jana, S.; Das, A.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Aceclofenac-loaded unsaturated esterified alginate/gellan gum microspheres: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2013, 57, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Ely, D.R.; Kleinebudde, P. The water binding behavior of κ-Carrageenan determined by three different methods. Pharm. Dev. Technol. 2009, 14, 249–258. [Google Scholar] [CrossRef]
- Günter, E.A.; Martynov, V.V.; Belozerov, V.S.; Martinson, E.A.; Litvinets, S.G. International Journal of Biological Macromolecules Characterization and swelling properties of composite gel microparticles based on the pectin and κ-carrageenan. Int. J. Biol. Macromol. 2020, 164, 2232–2239. [Google Scholar] [CrossRef]
- Youse, M.; Mahdi, S. Recent advances in application of different hydrocolloids in dairy products to improve their techno-functional properties. Trends Food Sci. Technol. 2019, 88, 468–483. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, J.; Zou, J.; Yuan, X.; Li, J.; Liang, H.; Zhan, F.; Li, B. International Journal of Biological Macromolecules Partial removal of acetyl groups in konjac glucomannan signi fi cantly improved the rheological properties and texture of konjac glucomannan and κ-carrageenan blends. Int. J. Biol. Macromol. 2019, 123, 1165–1171. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.M.S. International Journal of Biological Macromolecules Synergic formulation of onion peel quercetin loaded chitosan-cellulose hydrogel with green zinc oxide nanoparticles towards controlled release, biocompatibility, antimicrobial and anticancer activity. Int. J. Biol. Macromol. 2019, 132, 784–794. [Google Scholar] [CrossRef]
- Nurman, S.; Yulia, R.; Irmayanti; Noor, E.; Sunarti, T.C. The optimization of gel preparations using the active compounds of arabica coffee ground nanoparticles. Sci. Pharm. 2019, 87, 32. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Su, H.; Fang, L.; Tan, T. Superabsorbent hydrogels from poly(aspartic acid) with salt-, temperature- and pH-responsiveness properties. Polymer 2005, 46, 5368–5376. [Google Scholar] [CrossRef]
- Xia, W.; Ma, L.; Chen, X.; Li, X.; Zhang, Y. Physicochemical and structural properties of composite gels prepared with myofibrillar protein and lecithin at various ionic strengths. Food Hydrocoll. 2018, 82, 135–143. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Rabalski, I.; Hernandez, M.; L’Hocine, L.; Patterson, C.A.; Hucl, P. Effect of sodium chloride, sucrose, and xanthan gum on pasting properties and gel syneresis of hairless canary seed starch. Cereal Chem. 2019, 96, 908–919. [Google Scholar] [CrossRef]
- Dobosz, A.; Sikora, M.; Krystyjan, M.; Tomasik, P.; Lach, R.; Borczak, B.; Berski, W.; Lukasiewicz, M. Short- and long-term retrogradation of potato starches with varying amylose content. J. Sci. Food Agric. 2019, 99, 2393–2403. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Compressive textural attributes, opacity and syneresis of gels prepared from gellan, agar and their mixtures. J. Food Eng. 2011, 102, 287–292. [Google Scholar] [CrossRef]



| Parameter | F1 | F2 | F3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 1 | 3 | 5 | 7 | 1 | 3 | 5 | 7 | |
| pH | 7.28 | 7.29 | 7.34 | 7.34 | 4.7 | 5.37 | 5.4 | 5.31 | 5.72 | 5.95 | 6.09 | 5.98 |
| Viscosity (cPs) | 8.6 | 9.88 | 9.99 | 9.99 | 2.33 | 2.43 | 2.55 | 2.66 | 3.77 | 3.85 | 4.27 | 4.54 |
| Spreadability (cm) | 3 | 3.4 | 3.4 | 3.5 | 5 | 5.2 | 5.41 | 5.53 | 3.77 | 3.85 | 4.27 | 4.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardhana, Y.W.; Aanisah, N.; Sopyan, I.; Hendriani, R.; Chaerunisaa, A.Y. Gelling Power Alteration on Kappa-Carrageenan Dispersion through Esterification Method with Different Fatty Acid Saturation. Gels 2022, 8, 752. https://doi.org/10.3390/gels8110752
Wardhana YW, Aanisah N, Sopyan I, Hendriani R, Chaerunisaa AY. Gelling Power Alteration on Kappa-Carrageenan Dispersion through Esterification Method with Different Fatty Acid Saturation. Gels. 2022; 8(11):752. https://doi.org/10.3390/gels8110752
Chicago/Turabian StyleWardhana, Yoga W., Nuur Aanisah, Iyan Sopyan, Rini Hendriani, and Anis Y. Chaerunisaa. 2022. "Gelling Power Alteration on Kappa-Carrageenan Dispersion through Esterification Method with Different Fatty Acid Saturation" Gels 8, no. 11: 752. https://doi.org/10.3390/gels8110752