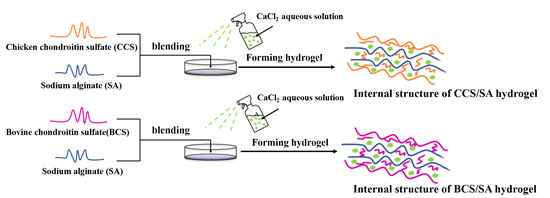
Fabrication and Characterization of Chicken- and Bovine-Derived Chondroitin Sulfate/Sodium Alginate Hybrid Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of CCS and BCS Particles
2.1.1. Particle Size Distribution
2.1.2. Transmission Electron Microscope (TEM) and Rheological Properties
2.1.3. Thermogravimetric Analysis (TGA)
2.1.4. Fourier Transform Infrared (FTIR)
2.2. Characterization of Hybrid Hydrogels
2.2.1. Evaporation Water Loss Rate
2.2.2. Swelling Rate
2.2.3. Thermogravimetric Analysis (TGA)
2.2.4. Fourier Transform Infrared (FTIR)
2.2.5. Texture Analysis
2.2.6. Microstructure Analysis
2.2.7. Low Field-Nuclear Magnetic Resonance (LF-NMR)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Characterization of CCS and BCS
4.2.1. Particle Size
4.2.2. Transmission Electron Microscopy (TEM)
4.2.3. Rheological Measurement
4.2.4. Thermogravimetric Analysis (TGA)
4.2.5. Fourier Transform Infrared Spectroscopy (FTIR)
4.3. Preparation of Hybrid Hydrogels
4.4. Characterization of Hybrid Hydrogels
4.4.1. Evaporation Water Loss Rate
4.4.2. Swelling Rate
4.4.3. Thermogravimetric Analysis (TGA)
4.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.4.5. Texture Analysis
4.4.6. Scanning Electron Microscopy (SEM)
4.4.7. Low-Field Nuclear Magnetic Resonance (LF-NMR)
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chivers, P.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef]
- Nunes, C.S.; Rufato, K.B.; Souza, P.R.; de Almeida, E.A.; da Silva, M.J.V.; Scariot, D.B.; Nakamura, C.V.; Rosa, F.A.; Martins, A.F.; Muniz, E.C. Chitosan/chondroitin sulfate hydrogels prepared in [Hmim][HSO4] ionic liquid. Carbohydr. Polym. 2017, 170, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Karoyo, A.H.; Wilson, L.D. Physicochemical properties and the gelation process of supramolecular hydrogels: A Review. Gels 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Simão, A.R.; Fragal, V.H.; Pellá, M.C.G.; Garcia, F.P.; Nakamura, C.V.; Silva, R.; Tambourgi, E.B.; Rubira, A.F. Drug polarity effect over the controlled release in casein and chondroitin sulfate-based hydrogels. Int. J. Biol. Macromol. 2020, 158, 116–126. [Google Scholar] [CrossRef]
- Fajardo, A.R.; Silva, M.B.; Lopes, L.C.; Piai, J.F.; Muniz, E.C. Hydrogel based on an alginate–Ca2+/chondroitin sulfate matrix as a potential colon-specific drug delivery system. RSC Adv. 2012, 2, 11095–11103. [Google Scholar] [CrossRef]
- Tang, C.; Holt, B.D.; Wright, Z.M.; Arnold, A.M.; Moy, A.C.; Sydlik, S.A. Injectable amine functionalized graphene and chondroitin sulfate hydrogel with potential for cartilage regeneration. J. Mater. Chem. B 2019, 7, 2442–2453. [Google Scholar] [CrossRef]
- Li, X.; Xu, Q.; Johnson, M.; Wang, X.; Lyu, J.; Li, Y.; McMahon, S.; Greiser, U.; Sigen, A.; Wang, W. A chondroitin sulfate based injectable hydrogel for delivery of stem cells in cartilage regeneration. Biomater. Sci. 2021, 9, 4127–4138. [Google Scholar] [CrossRef]
- Charbonneau, C.; Liberelle, B.T.; Hébert, M.; Crescenzo, G.D.; Lerouge, S. Stimulation of cell growth and resistance to apoptosis in vascular smooth muscle cells on a chondroitin sulfate/epidermal growth factor coating. Biomaterials 2011, 32, 1591–1600. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Parandeh, M.; Akbari, V.; Ebrahimi, Z.; Taheri, A. Incorporation of rosuvastatin-loaded chitosan/chondroitin sulfate nanoparticles into a thermosensitive hydrogel for bone tissue engineering: Preparation, characterization, and cellular behavior. Pharm. Dev. Technol. 2019, 24, 357–367. [Google Scholar] [PubMed]
- Kazi, G.A.; Yamamoto, O. Effectiveness of the sodium alginate as surgical sealant materials. Wound Med. 2019, 24, 18–23. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Sun, D.; Liu, Y.; Liu, Y.; Wang, Y.; Liu, C.; Wu, H.; Lv, Y.; Ren, Y.; et al. Development of a biomimetic chondroitin sulfate-modified hydrogel to enhance the metastasis of tumor cells. Sci. Rep. 2016, 6, 29858–29871. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Baruah, R.; Goyal, A. Physicochemical, antioxidant and biocompatible properties of chondroitin sulphate isolated from chicken keel bone for potential biomedical applications. Carbohydr. Polym. 2017, 159, 11–19. [Google Scholar] [CrossRef]
- Zou, Z.; Wei, M.; Fang, J.; Dai, W.; Sun, T.; Liu, Q.; Gong, G.; Liu, Y.; Song, S.; Ma, F.; et al. Preparation of chondroitin sulfates with different molecular weights from bovine nasal cartilage and their antioxidant activities. Int. J. Biol. Macromol. 2020, 152, 1047–1055. [Google Scholar] [CrossRef]
- Kakkar, P.; Madhan, B. Fabrication of keratin-silica hydrogel for biomedical applications. Mater. Sci. Eng. C 2016, 66, 178–184. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, C.; Mo, H.; Zhang, H.; Qin, X.; Li, J.; Zhang, Z.; Richel, A. Fabrication of chondroitin sulfate calcium complex and its chondrocyte proliferation in vitro. Carbohydr. Polym. 2021, 254, 117282–117292. [Google Scholar] [CrossRef]
- Garnjanagoonchorn, W.; Wongekalak, L.; Engkagul, A. Determination of chondroitin sulfate from different sources of cartilage. Chem. Eng. Process. Process Intensif. 2007, 46, 465–471. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Farran, A.; Montell, E.; Vergés, J.; Pelletier, J.P. Discrepancies in composition and biological effects of different formulations of chondroitin sulfate. Molecules 2015, 20, 4277–4289. [Google Scholar] [CrossRef]
- Ponsubha, S.; Jaiswal, A.K. Effect of interpolymer complex formation between chondroitin sulfate and chitosan-gelatin hydrogel on physico-chemical and rheological properties. Carbohydr. Polym. 2020, 238, 116179. [Google Scholar]
- Hu, X.; Li, D.; Zhou, F.; Gao, C. Biological hydrogel synthesized from hyaluronic acid, gelatin and chondroitin sulfate by click chemistry. Acta Biomater. 2011, 7, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Schuiringa, G.; Mihajlovic, M.; van Donkelaar, C.; Vermonden, T.; Ito, K. Creating a Functional Biomimetic Cartilage Implant Using Hydrogels Based on Methacrylated Chondroitin Sulfate and Hyaluronic Acid. Gels 2022, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.; Mohan, Y.M.; Sreeramulu, J.; Raju, K.M. Semi-IPNs of starch and poly(acrylamide-co-sodium methacrylate): Preparation, swelling and diffusion characteristics evaluation. React. Funct. Polym. 2006, 66, 1482–1493. [Google Scholar] [CrossRef]
- Ozel, B.; Uguz, S.S.; Kilercioglu, M.; Grunin, L.; Oztop, M.H. Effect of different polysaccharides on swelling of composite whey protein hydrogels: A low field (LF) NMR relaxometry study. J. Food Process Eng. 2017, 40, e12465. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Minhas, M.U.; Barkat, K. Synthesis and evaluation of chondroitin sulfate based hydrogels of loxoprofen with adjustable properties as controlled release carriers. Carbohydr. Polym. 2018, 181, 1169–1179. [Google Scholar] [CrossRef]
- Wang, L.F.; Shen, S.S.; Lu, S.C. Synthesis and characterization of chondroitin sulfate–methacrylate hydrogels. Carbohydr. Polym. 2003, 52, 389–396. [Google Scholar] [CrossRef]
- Fajardo, A.R.; Piai, J.F.; Rubira, A.F.; Muniz, E.C. Time- and pH-dependent self-rearrangement of a swollen polymer network based on polyelectrolytes complexes of chitosan/chondroitin sulfate. Carbohydr. Polym. 2010, 80, 934–943. [Google Scholar] [CrossRef]
- Crispim, E.G.; Piai, J.F.; Fajardo, A.R.; Ramos, E.R.F.; Nakamura, T.U.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Hydrogels based on chemically modified poly(vinyl alcohol) (PVA-GMA) and PVA-GMA/chondroitin sulfate: Preparation and characterization. Express Polym. Lett. 2012, 6, 383–395. [Google Scholar] [CrossRef]
- Ma, F.; Li, S.; Ruiz-Ortega, L.I.; Zhang, Y.; Tang, B. Effects of alginate/chondroitin sulfate-based hydrogels on bone defects healing. Mater. Sci. Eng. C 2020, 116, 111217. [Google Scholar]
- Lee, S.; Choi, J.; Youn, J.; Lee, Y.; Kim, W.; Choe, S.; Song, J.; Reis, R.; Khang, G. Development and Evaluation of Gellan Gum/Silk Fibroin/Chondroitin Sulfate T ernary Injectable Hydrogel for Cartilage Tissue Engineering. Biomolecules 2021, 11, 1184. [Google Scholar] [CrossRef]
- Han, L.; Wang, M.; Li, P.; Gan, D.; Yan, L.; Xu, J.; Wang, K.; Fang, L.; Chan, C.; Zhang, H.; et al. Mussel-inspired tissue adhesive hydrogel based on polydopamine-chondroitin sulfate complex for growth-factor-free cartilage regeneration. ACS Appl. Mater. Interfaces 2018, 10, 28015–28026. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Cevher, E.; Hatıpoğlu, F.; Oğurtan, Z.; Baş, A.L.; Akbuğa, J. Preparation of Fucoidan-Chitosan Hydrogel and Its Application as Burn Healing Accelerator on Rabbits. Biol. Pharm. Bull. 2008, 31, 2326–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, G.; Bidini, A.; Lamponi, S.; Magnani, A. States of water, surface and rheological characterisation of a new biohydrogel as articular cartilage substitute. Polym. Adv. Technol. 2013, 24, 824–833. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef]
- Fujiyabu, T.; Li, X.; Chung, U.I.; Sakai, T. Diffusion behavior of water molecules in hydrogels with controlled network structure. Macromolecules 2019, 52, 1923–1929. [Google Scholar] [CrossRef]
- Chen, F.; Chen, C.; Zhao, D.; Zhang, S.; Ma, G.; Su, Z.; Li, X. On-line monitoring of the sol-gel transition temperature of thermosensitive chitosan/β-glycerophosphate hydrogels by low field NMR. Carbohydr. Polym. 2020, 238, 116196. [Google Scholar] [CrossRef]
- Abasi, S.; Davis, R., Jr.; Podstawczyk, D.A.; Guiseppi-Elie, A. Distribution of water states within Poly(HEMA-co-HPMA)-based hydrogels. Polymer 2019, 185, 121978. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, Z.; Jing, P. Black rice anthocyanins embedded in self-assembled chitosan/chondroitin sulfate nanoparticles enhance apoptosis in HCT-116 cells. Food Chem. 2019, 301, 125280. [Google Scholar] [CrossRef]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Qu, Y.; Wang, B.; Sun, J.; Miao, C.; Zhang, C. Improving gelling properties of myofibrillar proteins incorporating with cellulose micro/nanofibres. Int. J. Food Sci. Technol. 2022, 57, 3428–3439. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, Y.; Lan, T.; Wang, B.; Huang, M.; Huang, H.; Qiao, C.; Sun, J. Fabrication and Characterization of Chicken- and Bovine-Derived Chondroitin Sulfate/Sodium Alginate Hybrid Hydrogels. Gels 2022, 8, 620. https://doi.org/10.3390/gels8100620
Zhao Y, Li Y, Lan T, Wang B, Huang M, Huang H, Qiao C, Sun J. Fabrication and Characterization of Chicken- and Bovine-Derived Chondroitin Sulfate/Sodium Alginate Hybrid Hydrogels. Gels. 2022; 8(10):620. https://doi.org/10.3390/gels8100620
Chicago/Turabian StyleZhao, Yaqi, Yan Li, Tianchan Lan, Baowei Wang, Ming Huang, He Huang, Changming Qiao, and Jingxin Sun. 2022. "Fabrication and Characterization of Chicken- and Bovine-Derived Chondroitin Sulfate/Sodium Alginate Hybrid Hydrogels" Gels 8, no. 10: 620. https://doi.org/10.3390/gels8100620