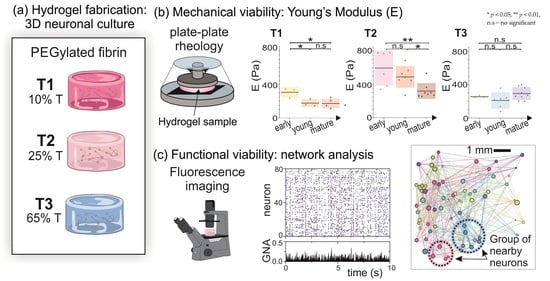
A total of three different PEGylated fibrin hydrogels were prepared with concentrations of 10%, 25%, and 65% in volume of thrombin. All hydrogel samples contain an ensemble of cortical neurons and glia cells that self-organize into a living neuronal network (
Figure 1a), and will be referred to as T1, T2, and T3 PEGylated fibrin, respectively, (
Figure 1b). We note that the mechanical characterization of T2 hydrogels was previously examined in a publication from our group [
17]. In order to establish a relationship between the mechanical properties of the hydrogel scaffolds and the functional organization of the resultant neuronal network (
Figure 1c), we present first the rheological characterization of the hydrogels with neurons, and then describe their functional organization as a living neuronal network.
2.1. Rheological Characterization of Hydrogels
We followed the protocol described in López-León et al. [
17] with minor modifications, as detailed in Methods. Hydrogels were first studied at day in vitro (DIV) 1, i.e., 24 h after culturing the cells, and their mechanical properties were monitored every 4–5 days up to 3 weeks. All hydrogels were studied using SAOS (Small Amplitude Oscillatory Shearing) rheology, as it provides direct information on the bulk mechanical properties of the material [
18,
19] (
Figure 1b). Three types of rheological tests were carried out to characterize the different samples:
time sweep, to evaluate the hydrogel stability during a 15 min measurement at controlled shear deformation,
, with
being the amplitude strain and
the frequency of the oscillation;
strain sweep, to characterize the hydrogel response to a varying amplitude strain
with a fixed frequency
; and
frequency sweep, to assess the hydrogel response to a varying frequency
and fixed amplitude strain
. All measurements were conducted on a Discovery HR-2 rheometer at a temperature of 37 °C. Since the instrument has a lower measurable limit of 2 nN·m of the oscillation torque, some extracted values of
and
from the strain sweep test were considered unreliable and therefore discarded.
2.1.1. Characterization of Hydrogels Viscoelastic Behavior
Figure 2 shows the results for the time sweep tests applied to the T1, T2 and T3 samples at DIV 1. Data points were obtained after averaging three different repetitions with the corresponding standard error. The rheological measurements show a stable response of the hydrogels under this test after a transient of
s for T1 and T3 samples and
s for T2 hydrogels (black arrowheads). T2 hydrogels took longer times to reach an equilibrium state after been subjected to an oscillatory deformation. Despite the difference in the duration of this transient regime, all hydrogels showed a reasonable reproducibility with a consistent
throughout the mechanical test. The fact that
is an order of magnitude larger than
means that the elastic contribution of the hydrogel dominates over the viscous one.
Strain sweep tests were performed at DIV 1 to investigate the mechanical response of the hydrogels with the amplitude of strain. Left panels of
Figure 3a–c show
and
for all hydrogel types (T1, T2 and T3). These plots clearly show a slight negative trend of
as the strain amplitude
is increased, indicating that one cannot fully consider the system to be in the linear viscoelastic regime. Nevertheless, the dependence on
was minimal. To facilitate the interpretation of results, we assume that any variations in the sample were minor, i.e., the tests did not alter the structure of the sample. Thus, we fixed
to perform the frequency test sweep on the different hydrogels. Additionally, since
and
do not cross each other in the whole range of
investigated, we expect that the qualitative behavior of
and
is not affected by the choice of
.
The frequency test was used to characterize changes in the structure of the hydrogel since it gives information about the crosslinking degree of the material. Results are shown in the right panels of
Figure 3a–c. The behavior of the
and
curves are typical for a hydrogel structure [
20], with
for all frequencies and both moduli displaying a plateau at oscillation frequencies
rad/s, evidence of the successful formation of a stable crosslinked network [
19]. Results provided
Pa for T1 and T3 hydrogels and
Pa for T2 ones. Therefore,
, indicating that the larger amount of thrombin in T2 stiffened the scaffold. However, we unexpectedly measured
. To understand this result, we note that Weisel et al. [
21] reported that maximal stiffness is found for fibrin gels that display fiber lengths, diameters, and branch point densities that are intermediate in magnitude, which provides a justification for the observed
.
2.1.2. Evolution of T1, T2 and T3 Hydrogels during Development
To test the validity of T1, T2 and T3 PEGylated fibrin hydrogels as scaffolds for neuronal cultures, we studied the evolution of their mechanical properties and stability during neuronal network development, from DIV 1 to 20. Since
exceeded
by a decade (see
Figure 3), we only show plots for
in the following analyses.
Results for the strain sweep test for T1, T2 and T3 samples from DIV 1 to DIV 20 are shown in the left panels of
Figure 4a–c. T1 hydrogels exhibited the largest variability in
values across different DIVs.
values oscillated in the range 80–290 Pa with curves that crossed one other and with an evident drift as the amplitude strain
grew. For T2 hydrogels,
was within a range of 140–300 Pa, presenting less sparsity across different DIVs. Finally, T3 exhibited the most similar behavior across DIVs (except at DIV 8), with a
value in the range of 150–200 Pa. The minimal dispersion of curves indicates that the hydrogel matrix became highly compact independently of the DIV, suggesting that neurons were not extending axons throughout the material and therefore they did not alter the hydrogel structure. Complementary, we observed that these T3 hydrogels failed at showing neuronal activity.
The right panels of
Figure 4a–c display the results obtained for the frequency sweep tests conducted at
= 5%. It is worth noticing that the gel-characteristic low-frequency plateau was present in all the samples at different DIVs. However, we observed differences in the behavior exhibited by the samples, with the plateau regime ending at frequencies of
rad/s for T1 and T3 hydrogels, and extending to approximately
rad/s for the T2 ones.
In addition, the strong thickening —associated to an abrupt hardening of the material— of the hydrogels at large frequencies (
rad/s) was more pronounced for T1 (lowest thrombin concentration) and T3 (highest) than for T2 (intermediate). This thickening at high-frequency oscillations could be related to the failure of the network of the gel to rearrange when subjected to fast oscillations, i.e., short time-scale perturbations [
22]. However, this effect might be also caused by high-frequency perturbations that induce inertia effects in the response of the hydrogels [
23,
24]. Intermediate thrombin concentrations, as in T2, were reported to induce fibrin networks with higher stiffness and resilience due to an increased crosslinking density [
6,
25]. Thus, it is plausible that the polymeric network of T1 and T3 exhibited diminished resistance, leading to an earlier decline in the capability of their polymer chains to rearrange when subjected to fast oscillations as compared to T2, which could be considered optimal. Another plausible scenario is that, due to the comparatively lower stiffness of the T1 and T3 structures, inertia effects triggered at high frequencies could exert a more pronounced impact on the resulting curves.
The Young’s modulus (
E) for the three variants of PEGylated fibrin, was calculated by using Equations (
2) and (3). Results in
Figure 5 show the distribution of
E values for three evolutionary stages (early ‘e’, young ‘y’ and mature ‘m’). The mean value of
E for each stage is indicated by a black horizontal line. Color boxes indicate the standard deviation of the samples. The evolution of
E for T1 hydrogels (
Figure 5a) showed a significant evolution from the early stage of development (
Pa) to the young one, but there was no a significant difference between the young and mature stages, in which
Pa in both cases (
). Results for T2 are also shown in
Figure 5b. The mean value of
E within each evolutionary stage gradually decayed, from
Pa at the early developmental stage to
Pa at the mature one, a decrease of practically a factor of two that was statistically significant (
). We postulate that this loss of elasticity is caused by the presence of developing neurons within the structure. On the other hand,
E for T3 hydrogels (
Figure 5c) did not show a clear evolution with time, with
Pa on average for all developmental stages with no significant variations (
). This apparent stability could be attributed to the lack of living neurons that kept the gel unperturbed.
Overall, for all the developmental stages, T2 presented the highest
E, indicating that intermediate levels of thrombin are actually those that produce the stiffest structure, and with viable neurons within. Lower amounts of thrombin (T1 hydrogels) led to softer structures with living neurons, while higher amounts of thrombin (T3) led to hydrogels that could not sustain the development of neurons. Indeed, since these T3 samples contain the highest concentration of thrombin, we conjecture that neurons did not survive the gelation process of the scaffold, indicating that there is a limit in thrombin concentration above which neurons are not able to survive. Indeed, the failed network in T3 is possibly due to the formation of a highly compact environment of fibrin fibers, with pores so small that cannot accommodate healthy neurons. Our observation is in agreement with a number of studies in the literature pointing out that pore size is crucial for the healthy formation of a neuronal network [
1].
2.2. Functional Characterization of Hydrogels’ Neuronal Networks
The above results showed that T1 and T2 hydrogels were clearly different in overall stiffness but, nonetheless, both provided a healthy environment for neurons to develop. In an attempt to relate the mechanical properties of the hydrogels with functional characteristics of the generated neuronal networks, we monitored spontaneous activity in the hydrogels and extracted information such as overall dynamic behavior and network communication. Since T3 samples were not viable as a living network, they were omitted from this functional analysis.
As described in Methods, neurons were transduced with the fluorescence calcium indicator GCaMP6s during the preparation of the hydrogels, allowing to monitor neuronal spontaneous activity from DIV 7 onwards. Activity was recorded for 15 min every two days and included on the order of 100 neurons. The optical system acquired neuronal activity on a single focus plane of the 3D structure, as sketched in
Figure 6, and therefore the monitored area must be considered as a proxy of the entire system. The central panels of
Figure 6 provide characteristic fluorescence images at the focus plane for the T1 and T2 hydrogels. In both cases, neurons are clearly visible as bright circular spots.
The right panels of
Figure 6 show representative raster plots of recorded spontaneous activity at DIV 15, a mature stage in the networks, and for the T1 (
Figure 6a) and T2 (
Figure 6b) hydrogels. Activity was abundant in both cases, although the soft T1 hydrogel was slightly more active (
activations per neuron and minute) than the stiff T2 one (
activations). Additionally, T1 exhibited a clear tendency towards stronger synchronization as compared to T2, as revealed by the population activity panels below the raster plots. The population activity counts the fraction of neurons in the network that coactivate in a short time window. For the T1 hydrogel, we can observe strong peaks (marked with yellow arrows) that reveal episodes of neuronal coordinated activity, which are absent in the T2 hydrogel.
We next applied Transfer Entropy (TE) to the activity data to gain information on the functional organization of the neuronal networks, i.e., the degree of communication of neurons within the hydrogel. As described in Methods, we considered three main metrics, namely the average connectivity
, which accounts for the average communication links of a neuron with others; the global efficiency (
), which describes the easiness for information to flow across the network; and the modularity (
Q), which informs about the tendency of neurons to preferentially interact in small groups (termed
communities) rather than as a whole system. These metrics are widely used to evaluate and quantify functional differences between networks or contrasting states within the same network, e.g., in disease models [
26], neuroengineering [
27] or development [
28]. The repertoire of possible metrics is abundant is and applicable to all fields in which complex networks are central [
29,
30].
The left panels of
Figure 7 show the effective connectivity matrices, procured by TE analysis, and for the same data as in
Figure 6. Black dots in these matrices indicate a significant effective connection between neuronal pairs, while the color boxes indicate communities of neurons that tend to communicate more strongly within their group than with the rest of the network. The right panels of
Figure 7 show the corresponding connectivity maps, with neurons placed as they appeared in the images and colored according to the community they belong to.
As a first difference between the two preparations, the soft T1 hydrogels had an average connectivity of
connections per neuron, higher than the stiff T2 hydrogel with
connections per neuron. This higher average connectivity of T1 is expected given its strongest synchronization and that translates into a higher communication of neurons across the network. The overall strong network cohesion of T1 is also reflected by the global efficiency
, the bulk network property that captures the flow of information in the network, with
for T1 and
for T2, i.e, neurons in T1 were about 15% more efficient communicating across the network. On the other hand, both hydrogels exhibited practically the same modularity
, indicating that neurons tended to form functional communities in a similar way. This means that, despite the higher synchronization of T1 hydrogels, neurons in both preparations maintained specialized communication at a local scale. Interestingly, as shown in the T1 network map of
Figure 7a, neurons within a community (particularly the pink and blue) were physically nearby, possibly reflecting the presence of short axons that mediated local communication. In turn, these short axons were combined with longer ones that facilitated whole network synchronization. Such a physical proximity of neurons within a community is not so clear in the T2 hydrogels (
Figure 7b). We hypothesize that axons could be longer here, but that they did not orchestrate abundant synchronization possibly due to the observed low connectivity.
2.3. Discussion: Influence of Hydrogel Composition and Microstructure on Young’s Modulus
Free thrombin concentration at the time of gelation influences fibrin hydrogel structure [
8,
9]. Low concentrations of thrombin lead to fibrin gels formed by thick fibers, few branch points and large pores, while higher concentrations lead to gels consisting of tightly packed thin fibers. Taking these factors into consideration, one would expect that thrombin concentration correlates with the stiffness of the generated hydrogel since a network of loosely entangled fibers have less stiffness than a network of densely compacted ones, with the latter better resisting the application of an external force or deformation strain. With the goal of assessing whether such a correlation holds, we characterized the mechanical behavior of T1, T2 and T3 PEGylated fibrin samples along a three-week period, and observed that
<
, for every developmental stage, suggesting at a first glance that hydrogels with a greater amount of thrombin exhibited a higher stiffness. However, we also observed that
<
, which is initially puzzling but that can be explained in the context of the work by Weisel et al. [
21]. These authors reported that maximal stiffness is found for fibrin gels that display fiber lengths, diameters, and density of branch points that are intermediate in magnitude, i.e., high stiffness stems from a balance between an increased level of branching and thicker fibers, attributes that enhance network rigidity. Thus, in our study, very high thrombin concentrations did not make a stiffer hydrogel but a structure that was unsuitable as a biomaterial.
The brain Young’s modulus is
Pa after birth, and increases as neurons develop to about
kPa in adulthood. This change can be understood in the context of the study by Spedden and coworkers [
31] who described that stiffness of cortical networks increases with maturation as a result of the stabilization of neuron’s microtubule cytoskeleton. The range of values for
is compatible with our measurements in the T1–T3 hydrogels, with
E typically varying in the range 200–600 Pa. However, our data has the opposite trend than the brain, with
E decreasing with development, suggesting a potential degradation of PEGylated fibrin that concurs with neuronal network formation. In this regard, Berkovitch et al. [
32], conducted a study comparing the mechanical properties of semi-synthetic, cell-free hydrogels made from PEG and various natural protein combinations, including fibrinogen. They assessed hydrogel density during incubation with culture medium, calculated in relation to the corresponding dry weight of samples. Their results showed a decrease in hydrogel density over time for all materials. Such a degradation process, in the absence of cells, arises from enzymes such as proteases present in the culture medium, leading to enzymatic and hydrolytic degradation of the hydrogels. Thus, it is possible that two opposing mechanisms are at play in our hydrogels, one related to neuronal network formation that increases
E, and another one related to enzymatic action that decreases
E, with the latter possibly being dominant.
In line of the above arguments, it is clear that the initial mechanical properties of the hydrogel and degradation kinetics are crucial for determining the capacity of neurons to survive and grow within the hydrogel. In physiological conditions,
fibrinolysis, i.e., the degradation of fibrin clots, is mainly governed by the serine-protease
plasmin, which is derived from its inactive precursor, plasminogen. This conversion is usually triggered by substances like tissue plasminogen activator (tPA) or urokinase plasminogen activator (uPA) [
10]. The architecture of fibrin gel has an impact on the rates of fibrinolysis [
33], although the mechanisms involved are not fully understood yet. Studies have revealed that fibrin matrices constituted by thicker fibers (low thrombin concentrations) tend to undergo faster lysis than those containing thinner fibrin fibers (high thrombin concentrations) [
8,
21,
34,
35]. However, by contrast, thin fibers are actually lysed faster than thick ones since tPA diffuses more rapidly through a loose fibrin network than on a tight one [
36]. Thus, considering all these mechanisms together, the fall of
E along development shown in
Figure 5 can be explained in terms of the degradation of fibrin structure.
2.4. Discussion: Hydrogel Stability and Network Formation
Neurons and other cells in the central nervous system express tPA to promote synaptic plasticity and other neurophysiological processes [
37,
38,
39], which can accelerate the degradation of fibrin gels in vitro. We would therefore expect that our T2 hydrogels, made of a balanced structure of thin yet compact fibers, should experience a slower degradation process than the highly porous T1 ones, where tPA generated by neurons could travel along the volume faster. However, results showed the opposite trend, with the degradation process occurring slowly for the T1 hydrogels, in which
did not change significantly during the first 4 days of monitoring (
Figure 5). We conjecture that the larger pore size of these hydrogels reduced the capacity of axons to extend, slowing down network formation, maturation, and the production of metabolic factors. Additional investigations would be required to fully understand the mechanisms involved in hydrogel degradation, which are beyond the scope of this work. For instance, biochemical and transcriptomic analyses could help monitoring the expression of enzymes or proteins with potential degradation actions, such as the recent work by Zhou et al. [
40]. Additionally, imaging techniques like SEM would be needed to fully characterize the microstructure of the hydrogels and its impact on network formation. Without this information we cannot extract further conclusions on the internal dynamics within the hydrogels and the possible mechanisms that are governing their degradation. Regarding T3 hydrogels, we measured an approximately constant stiffness along the 20 days of tracking with
Pa. This result may be attributed to the absence of living cells within its structure. This hypothesis is supported by our previous work [
17], where we examined the evolution of T2–like hydrogels with and without cells. For matrices without cells we measured a Young’s modulus of
Pa that did not change during development. Thus, we argue that the observed
Pa along three weeks reflects a hydrogel without developing neurons.
We note that thrombin concentration not only regulates the stiffness and architecture of the fibrin structure, but also the stability of the gel when used for culturing cells. Tissue stiffness determines the length of neuronal axons and their degree of spreading [
41,
42,
43]. Therefore, the mechanical characterization of hydrogels is in general important to understand the behavior and emerging functional organization of the neuronal network growing within them. Koser et al. [
14], for instance, reported in a study on 2D cultures that a matrix with high stiffness induced persistent growth of axons, facilitating fasciculation (parallel growth of axons), whereas a matrix with low stiffness promoted a slowed exploratory growth with splaying of axons that led to a cross-linked, well-connected network. Our observation that the relatively soft T1 hydrogels have a higher global efficiency and connectivity, with clear episodes of synchronized activity, as compared to the stiffer T2 ones may be due to the capacity of T1 neurons to extend more axons—though shorter—in their neighborhoods, increasing overall network cohesiveness. However, these arguments are speculative since we lack direct structural information of the neuronal network, e.g., through SEM or 3D immunostaining analyses, which are by themselves difficult due the fragility of the hydrogels and the difficulty for molecules and antibodies to penetrate the 3D environment.
The ability of axons to spread across the hydrogel environment is the key determinant to orchestrate a viable, living network able to display coordinated activity [
44]. However, in general terms, two antagonistic mechanisms come into play when evaluating the capacity of such axons to spread, namely stiffness and porosity. Stiffness was introduced above in the context of Koser et al. [
14] and essentially governs the growth of axons as straight filaments or branch-out processes. Porosity, however, dictates the available space and the capacity of neurons to move and accommodate in the environment. Big pores would facilitate neuronal aggregation and the easy extension of neurites in all directions, but may result in few anchoring sites and an overall weak structural integrity. Conversely, small pores would substantially limit the spreading of neurons and block their signaling, but would strengthen their anchoring and overall structural integrity. These two mechanisms may be continuously at play during the formation of the living neuronal network in a highly complex manner, making very difficult to predict its dynamical or functional characteristics just from the main bulk ingredients of the prepared hydrogel.
It is also important to highlight that, within the brain, a wealth of genetic mechanisms regulate neuronal migration and differentiation as well as coarse axonal guidance [
45,
46], which leads to brain regions with substantial structural differences and mechanical properties [
47]. Such a guided network formation is present in vitro only when hydrogels are combined with microfluidics technology [
48,
49,
50,
51]. For instance, Cosson et al. designed a hydrogel microfluidic culture platform able to spatiotemporally control neuronal commitment of mouse embryonic stem cells through a gradient of retinoic acid, shaping a network with different kinds of cells or developmental stages [
52]. In this and other studies, an important limitation when extrapolating network formation in hydrogels to the brain is that the latter uses richly spatiotemporal sensory input to tune connectivity and refine neuronal circuitry towards task-specific functions [
53]. However, to the best of our knowledge, there are no in vitro studies that combine hydrogels, mircofluidics, input stimulation, and network connectivity analysis. The latter, as in our case, would be a certainly important ingredient to relate the dictated structural organization with activity and function. This is particular important for regenerative medicine, where functional tissue replacement and directed stem cell fate are central [
54,
55]. In this regard, the hydrogel that we introduced, in its various variations, primarily consists of the natural ingredients thrombin and fibrinogen, which are biodegradable and can be harvested from the same donor of specific cells or tissues. Thus, hydrogels like ours hold significant relevance for personalized applications, as utilizing components derived from patients ensure that implanted tissues are free from rejection [
56].
Keeping in mind the constraints on pore size and neurite extension, our T1 hydrogels were characterized by a lower stiffness and a higher porosity as compared to T2 ones. Based on the results shown in
Figure 7, we hypothesize the following scenario. Neurons within the T1 hydrogels had a higher capacity to spread axons across local neighborhoods, facilitating the intercommunication of nearby neurons, as observed in the functional analysis and that reveals communities that are formed by physically close neurons. Also, the easy spread of connections increases overall network cohesion that translates into a higher average connectivity and global efficiency (both about 16% higher in T1 than in T2), ultimately facilitating the emergence of network synchronous events. Interestingly, the T1 network exhibits a balance of local properties (well connected neighborhoods), and global ones (synchronous activity), shaping a network that combines integrated and segregated characteristics, an aspect that is considered optimal for neuronal systems [
57]. For the T2 hydrogels, axons grow straighter with a lower capacity to establish a dense mesh of connections. Neurons interconnect, but the functional communities that they form encompass neurons that are relatively far from one another. The formation of long yet poorly branched axons leads to an overall reduced connectivity with weak collective activity. T2 is viable as a living system, but it lacks the functional richness that T1 offers.