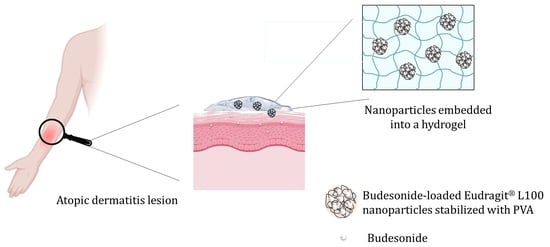
Formulation of Budesonide-Loaded Polymeric Nanoparticles into Hydrogels for Local Therapy of Atopic Dermatitis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of the Nanoparticles
2.2. Cytotoxicity Evaluation of HaCaT Cells
2.3. Preparation and Characterization of the Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Nanoparticles
4.3. Determination of Nanoparticle Size, Polydispersity Index (PDI), and Zeta-Potential
4.4. X-ray Powder Diffraction Analysis (XRPD) and FTIR Spectrophotometry
4.5. Cytotoxicity Evaluation of HaCaT Cells
4.6. Hydrogel Preparation
4.7. Appearance and pH of Hydrogels
4.8. In Vitro Occlusion Test
4.9. Rheology, Spreadability, and Penetrometry of Hydrogels
4.10. In Vitro Dissolution Test and Release Kinetics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suga, H.; Sato, S. Novel Topical and Systemic Therapies in Atopic Dermatitis. Immunol. Med. 2019, 42, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Behl, T.; Sharma, N.; Zahoor, I.; Chigurupati, S.; Yadav, S.; Rachamalla, M.; Sehgal, A.; Naved, T.; Pritima; et al. Targeting Therapeutic Approaches and Highlighting the Potential Role of Nanotechnology in Atopic Dermatitis. Environ. Sci. Pollut. Res. 2022, 29, 32605–32630. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Sahle, F.F.; Lohan, S.B.; Saeidpour, S.; Albrecht, S.; Teutloff, C.; Bodmeier, R.; Unbehauen, M.; Wolff, C.; Haag, R.; et al. pH-Sensitive Eudragit® L 100 Nanoparticles Promote Cutaneous Penetration and Drug Release on the Skin. J. Control. Release 2019, 295, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Padula, C.; Machado, I.P.; Vigato, A.A.; de Araujo, D.R. New Strategies for Improving Budesonide Skin Retention. Pharmaceutics 2021, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Almoshari, Y. Novel Hydrogels for Topical Applications: An Updated Comprehensive Review Based on Source. Gels 2022, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Davari, D.R.; Nieman, E.L.; McShane, D.B.; Morrell, D.S. Current Perspectives on the Management of Infantile Atopic Dermatitis. J. Asthma Allergy 2020, 13, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K.; Sherje, A.P. Nano Intervention in Topical Delivery of Corticosteroid for Psoriasis and Atopic Dermatitis-a Systematic Review. J. Mater. Sci. Mater. Med. 2021, 32, 88. [Google Scholar] [CrossRef]
- Kwatra, G.; Mukhopadhyay, S. Topical corticosteroids: Pharmacology. In A Treatise on Topical Corticosteroids in Dermatology: Use, Misuse and Abuse; Lahiri, K., Ed.; Springer: Singapore, 2018; pp. 11–22. ISBN 978-981-10-4609-4. [Google Scholar]
- Spada, F.; Barnes, T.M.; Greive, K.A. Comparative Safety and Efficacy of Topical Mometasone Furoate with Other Topical Corticosteroids. Australas. J. Dermatol. 2018, 59, e168–e174. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; da Costa, T.G.; de Lima, R.; Hedtrich, S.; Fraceto, L.F.; de Araujo, D.R. Hydrogels Containing Budesonide-Loaded Nanoparticles to Facilitate Percutaneous Absorption for Atopic Dermatitis Treatment Applications. ACS Appl. Polym. Mater. 2021, 3, 4436–4449. [Google Scholar] [CrossRef]
- Li, C.-Y.; Liu, Z. Effect of Budesonide on Hospitalization Rates among Children with Acute Asthma Attending Paediatric Emergency Department: A Systematic Review and Meta-Analysis. World J. Pediatr. WJP 2021, 17, 152–163. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, H. Preparation and Characterization of pH-Sensitive Nanoparticles of Budesonide for the Treatment of Ulcerative Colitis. Drug Des. Devel. Ther. 2018, 12, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Mota, F.L.; Carneiro, A.P.; Queimada, A.J.; Pinho, S.P.; Macedo, E.A. Temperature and Solvent Effects in the Solubility of Some Pharmaceutical Compounds: Measurements and Modeling. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2009, 37, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Daley-Yates, P.T. Inhaled Corticosteroids: Potency, Dose Equivalence and Therapeutic Index. Br. J. Clin. Pharmacol. 2015, 80, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Baek, H.H.; Lee, J.M.; Kim, J.H.; Kim, D.D.; Cho, H.K.; Cheong, I.W. Topical Delivery of Budesonide Emulsion Particles in the Presence of PEO-PCL-PEO Triblock Copolymers. Macromol. Res. 2009, 17, 969–975. [Google Scholar] [CrossRef]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and Nanofibers for Topical Drug Delivery. J. Control. Release Off. J. Control. Release Soc. 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Andrés Real, D.; Gagliano, A.; Sonsini, N.; Wicky, G.; Orzan, L.; Leonardi, D.; Salomon, C. Design and Optimization of pH-Sensitive Eudragit Nanoparticles for Improved Oral Delivery of Triclabendazole. Int. J. Pharm. 2022, 617, 121594. [Google Scholar] [CrossRef]
- Rowe, R.C. Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK, 2020. [Google Scholar]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical Significance of Eudragit: A Review. Future J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Sahle, F.F.; Balzus, B.; Gerecke, C.; Kleuser, B.; Bodmeier, R. Formulation and in Vitro Evaluation of Polymeric Enteric Nanoparticles as Dermal Carriers with pH-Dependent Targeting Potential. Eur. J. Pharm. Sci. 2016, 92, 98–109. [Google Scholar] [CrossRef]
- Cardoso, A.M.L.; Oliveira, E.E.; Machado, B.A.S.; Marcelino, H.R. Eudragit®-Based Nanoparticles for Controlled Release through Topical Use. J. Nanoparticle Res. 2023, 25, 32. [Google Scholar] [CrossRef]
- Menon, J.U.; Kona, S.; Wadajkar, A.S.; Desai, F.; Vadla, A.; Nguyen, K.T. Effects of Surfactants on the Properties of PLGA Nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation Enhancer Strategies in Transdermal Drug Delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Ference, J.D.; Last, A.R. Choosing Topical Corticosteroids. Am. Fam. Physician 2009, 79, 135–140. [Google Scholar] [PubMed]
- Coondoo, A.; Chattopadhyay, C. Use and Abuse of Topical Corticosteroids in Children. Indian J. Paediatr. Dermatol. 2014, 15, 1. [Google Scholar] [CrossRef]
- Cai, M.-H.; Chen, X.-Y.; Fu, L.-Q.; Du, W.-L.; Yang, X.; Mou, X.-Z.; Hu, P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef] [PubMed]
- Slavkova, M.; Tzankov, B.; Popova, T.; Voycheva, C. Gel Formulations for Topical Treatment of Skin Cancer: A Review. Gels 2023, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.P.; Spada, F. Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics 2018, 10, 71. [Google Scholar] [CrossRef]
- Cardoso, A.M.; de Oliveira, E.G.; Coradini, K.; Bruinsmann, F.A.; Aguirre, T.; Lorenzoni, R.; Barcelos, R.C.S.; Roversi, K.; Rossato, D.R.; Pohlmann, A.R.; et al. Chitosan Hydrogels Containing Nanoencapsulated Phenytoin for Cutaneous Use: Skin Permeation/Penetration and Efficacy in Wound Healing. Mater. Sci. Eng. C 2019, 96, 205–217. [Google Scholar] [CrossRef]
- Morsi, N.M.; Abdelbary, G.A.; Ahmed, M.A. Silver Sulfadiazine Based Cubosome Hydrogels for Topical Treatment of Burns: Development and in Vitro/in Vivo Characterization. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2014, 86, 178–189. [Google Scholar] [CrossRef]
- Marafon, P.; Fachel, F.N.S.; Dal Prá, M.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Development, Physico-Chemical Characterization and in-Vitro Studies of Hydrogels Containing Rosmarinic Acid-Loaded Nanoemulsion for Topical Application. J. Pharm. Pharmacol. 2019, 71, 1199–1208. [Google Scholar] [CrossRef]
- Wei, S.; Xie, J.; Luo, Y.; Ma, Y.; Tang, S.; Yue, P.; Yang, M. Hyaluronic Acid Based Nanocrystals Hydrogels for Enhanced Topical Delivery of Drug: A Case Study. Carbohydr. Polym. 2018, 202, 64–71. [Google Scholar] [CrossRef] [PubMed]
- González-Delgado, J.A.; Castro, P.M.; Machado, A.; Araújo, F.; Rodrigues, F.; Korsak, B.; Ferreira, M.; Tomé, J.P.C.; Sarmento, B. Hydrogels Containing Porphyrin-Loaded Nanoparticles for Topical Photodynamic Applications. Int. J. Pharm. 2016, 510, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Yasasvini, S.; Anusa, R.S.; VedhaHari, B.N.; Prabhu, P.C.; RamyaDevi, D. Topical Hydrogel Matrix Loaded with Simvastatin Microparticles for Enhanced Wound Healing Activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P. Excipients for semisolid formulations. In Excipient Development for Pharmaceutical, Biotechnology, and Drug Delivery Systems; Taylor & Francis Group: Oxford, UK, 2006; p. 197. [Google Scholar]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Influence of Molar Mass and Concentration on the Thermogelation of Methylcelluloses. Int. J. Polym. Anal. Charact. 2015, 20, 110–118. [Google Scholar] [CrossRef]
- Mut, A.M.; Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.I.; Stănciulescu, C.O.; Mitu, M.A.; Szabadai, Z.; Lupuleasa, D. Chitosan/Hpmc-Based Hydrogels Containing Essential Oils for Topical Delivery of Fluconazole: Preliminary Studies. Farmacia 2018, 66, 248–256. [Google Scholar]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse Effects of Topical Glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef]
- Saadallah, M.S.; Hamid, O.A. Eudragit® L100 Nanoparticles for Transdermal Delivery of Rosuvastatin Calcium. J. Excip. Food Chem. 2022, 13, 80–93. [Google Scholar]
- Duman, O.; Tunç, S. Electrokinetic and Rheological Properties of Na-Bentonite in Some Electrolyte Solutions. Microporous Mesoporous Mater. 2009, 117, 331–338. [Google Scholar] [CrossRef]
- Cetin, M.; Atila, A.; Kadioglu, Y. Formulation and in Vitro Characterization of Eudragit® L100 and Eudragit® L100-PLGA Nanoparticles Containing Diclofenac Sodium. AAPS PharmSciTech 2010, 11, 1250–1256. [Google Scholar] [CrossRef]
- Torres-Flores, G.; Türeli Nazende, G.; Akif Emre, T. Preparation of Fenofibrate Loaded Eudragit L100 Nanoparticles by Nanoprecipitation Method. Mater. Today Proc. 2019, 13, 428–435. [Google Scholar] [CrossRef]
- Albertsson, J.; Oskarsson, Å.; Svensson, C. X-Ray Study of Budesonide: Molecular Structures and Solid Solutions of the (22S) and (22R) Epimers of 11β, 21-Dihydroxy-16α, 17α-Propylmethylenedioxy-1, 4-Pregnadiene-3, 20-Dione. Acta Crystallogr. B 1978, 34, 3027–3036. [Google Scholar] [CrossRef]
- Nikam, A.; Sahoo, P.R.; Musale, S.; Pagar, R.R.; Paiva-Santos, A.C.; Giram, P.S. A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare. Pharmaceutics 2023, 15, 587. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yu, D.; Zhu, L.; Branford-White, C.; White, K.; Chatterton, N.P. Electrospun Diclofenac Sodium Loaded Eudragit® L 100-55 Nanofibers for Colon-Targeted Drug Delivery. Int. J. Pharm. 2011, 408, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.; Mitjans, M.; Infante, M.R.; Vinardell, M.P. Potential Irritation of Lysine Derivative Surfactants by Hemolysis and HaCaT Cell Viability. Toxicol. Lett. 2006, 161, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Armenise, N.; Bigucci, F.; Cerchiara, T.; Gösser, M.B.; Samorì, C.; Galletti, P.; Tagliavini, E.; Brown, D.M.; Johnston, H.J.; et al. Surfactants from Itaconic Acid: Toxicity to HaCaT Keratinocytes in Vitro, Micellar Solubilization, and Skin Permeation Enhancement of Hydrocortisone. Int. J. Pharm. 2017, 524, 9–15. [Google Scholar] [CrossRef]
- Li, H.; Toh, P.Z.; Tan, J.Y.; Zin, M.T.; Lee, C.-Y.; Li, B.; Leolukman, M.; Bao, H.; Kang, L. Selected Biomarkers Revealed Potential Skin Toxicity Caused by Certain Copper Compounds. Sci. Rep. 2016, 6, 37664. [Google Scholar] [CrossRef]
- Wilheilm, K.P.; Samblebe, M.; Siegers, C.P. Quantitative in Vitro Assessment of N-Alkyl Sulphate-Induced Cytotoxicity in Human Keratinocytes (HaCaT). Comparison with in Vivo Human Irritation Tests. Br. J. Dermatol. 1994, 130, 18–23. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Coughlin, M.L.; Liberman, L.; Ertem, S.P.; Edmund, J.; Bates, F.S.; Lodge, T.P. Methyl Cellulose Solutions and Gels: Fibril Formation and Gelation Properties. Prog. Polym. Sci. 2021, 112, 101324. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S.; Al-Dhubiab, B.; Attimarad, M.; Harsha, S. Basic Considerations in the Dermatokinetics of Topical Formulations. Braz. J. Pharm. Sci. 2013, 49, 423–434. [Google Scholar] [CrossRef]
- Li, L.; Thangamathesvaran, P.M.; Yue, C.Y.; Tam, K.C.; Hu, X.; Lam, Y.C. Gel Network Structure of Methylcellulose in Water. Langmuir 2001, 17, 8062–8068. [Google Scholar] [CrossRef]
- Hyun, K.; Nam, J.G.; Wilhellm, M.; Ahn, K.H.; Lee, S.J. Large Amplitude Oscillatory Shear Behavior of PEO-PPO-PEO Triblock Copolymer Solutions. Rheol. Acta 2006, 45, 239–249. [Google Scholar] [CrossRef]
- Pinto, J.R.; Monteiro e Silva, S.A.; de Holsback, V.S.S.; Leonardi, G.R. Skin Occlusive Performance: Sustainable Alternatives for Petrolatum in Skincare Formulations. J. Cosmet. Dermatol. 2022, 21, 4775–4780. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics 2017, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Op’t Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A.D.T.G. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-S.; Cho, H.-I.; Lee, H.-Y.; Lee, S.-H.; Choi, Y.-W. Enhanced Occlusiveness of Nanostructured Lipid Carrier (NLC)-Based Carbogel as a Skin Moisturizing Vehicle. J. Pharm. Investig. 2010, 40, 373–378. [Google Scholar] [CrossRef]
- Noval, N.; Rosyifa, R.; Annisa, A. Effect of HPMC concentration variation as gelling agent on physical stability of formulation gel ethanol extract bundung plants (Actinuscirpus Grossus). In Proceedings of the First National Seminar Universitas Sari Mulia (NS-UNISM 2019), Banjarmasin, Indonesia, 23 November 2019. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Torres, T.; Lima, S.A.C.; Reis, S. Hydrogels: A Promising Vehicle for the Topical Management of Atopic Dermatitis. Adv. Ther. 2021, 4, 2100028. [Google Scholar] [CrossRef]
- Sipos, E.; Szász, N.; Vancea, S.; Ciurba, A. Evaluation and Selection of Gel Base for the Formulation of Dexpanthenol Products. Trop. J. Pharm. Res. 2015, 13, 1987. [Google Scholar] [CrossRef]
- Helal, D.A.; El-Rhman, D.A.; Abdel-Halim, S.A.; El-Nabarawi, M.A. Formulation and Evaluation of Fluconazole Topical Gel. Int J Pharm Pharm Sci 2012, 4, 176–183. [Google Scholar]
- Levy, G.; Schwarz, T.W. The Effect of Certain Additives on the Gel Point of Methylcellulose. J. Am. Pharm. Assoc. Sci. Ed 1958, 47, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.; Lupuleasa, D. Cellulose-Derivatives-Based Hydrogels as Vehicles for Dermal and Transdermal Drug Delivery. Emerg. Concepts Anal. Appl. Hydrogels 2016, 2, 64. [Google Scholar]
- Zilberman, E.N.; Lerner, F.; Joseph, H.M.; Alon, M. Properties of Hydroxypropyl Methylcellulose–Polyvinyl Alcohol Water Systems, Dispersants in Vinyl Chloride Suspension Polymerization. J. Appl. Polym. Sci. 1993, 48, 435–442. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk-Soczynska, B.; Sajkiewicz, P.; Gradys, A. Toward a Better Understanding of the Gelation Mechanism of Methylcellulose via Systematic DSC Studies. Polymers 2022, 14, 1810. [Google Scholar] [CrossRef]
- Punitha, S.; Uvarani, R.; Panneerselvam, A. Effect of pH in Aqueous (Hydroxy Propyl Methyl Cellulose) Polymer Solution. Results Mater. 2020, 7, 100120. [Google Scholar] [CrossRef]
- Abdeltawab, H.; Svirskis, D.; Sharma, M. Formulation Strategies to Modulate Drug Release from Poloxamer Based in Situ Gelling Systems. Expert Opin. Drug Deliv. 2020, 17, 495–509. [Google Scholar] [CrossRef]
- Gandhi, A.; Jana, S.; Sen, K.K. In-Vitro Release of Acyclovir Loaded Eudragit RLPO® Nanoparticles for Sustained Drug Delivery. Int. J. Biol. Macromol. 2014, 67, 478–482. [Google Scholar] [CrossRef]
- Das, S.; Suresh, P.K.; Desmukh, R. Design of Eudragit RL 100 Nanoparticles by Nanoprecipitation Method for Ocular Drug Delivery. Nanomedicine Nanotechnol. Biol. Med. 2010, 6, 318–323. [Google Scholar] [CrossRef]
- Inal, O.; Yapar, E.A. Effect of Mechanical Properties on the Release of Meloxicam from Poloxamer Gel Bases. Indian J. Pharm. Sci. 2013, 75, 700–706. [Google Scholar]
- Zhang, L.; Parsons, D.L.; Navarre, C.; Kompella, U.B. Development and In-Vitro Evaluation of Sustained Release Poloxamer 407 (P407) Gel Formulations of Ceftiofur. J. Control. Release 2002, 85, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Musial, W. The Effect of Methylcellulose on Metronidazole Release from Polyacrylic Acid Hydrogels. Chem. Pharm. Bull. 2007, 55, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Bonina, F.P.; Montenegro, L. Vehicle Effects on in Vitro Heparin Release and Skin Penetration from Different Gels. Int. J. Pharm. 1994, 102, 19–24. [Google Scholar] [CrossRef]
- Tas, Ç.; Özkan, Y.; Savaser, A.; Baykara, T. In Vitro Release Studies of Chlorpheniramine Maleate from Gels Prepared by Different Cellulose Derivatives. Il Farm. 2003, 58, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Basak, R.; Bandyopadhyay, R. Encapsulation of Hydrophobic Drugs in Pluronic F127 Micelles: Effects of Drug Hydrophobicity, Solution Temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Joshi, V.; Yashaswini, G.; Acharya, A.; Bheemachari; Annegowda, H.V.; Niraula, B. Formulation and Evaluation of Semisolid Dosage Forms of an Anti-Inflammatory Drug. 3 Biotech 2019, 9, 248. [Google Scholar] [CrossRef]
- Batista, C.M.; de Queiroz, L.A.; Alves, Â.V.F.; Reis, E.C.A.; Santos, F.A.; Castro, T.N.; Lima, B.S.; Araújo, A.A.S.; Godoy, C.A.P.; Severino, P.; et al. Photoprotection and Skin Irritation Effect of Hydrogels Containing Hydroalcoholic Extract of Red Propolis: A Natural Pathway against Skin Cancer. Heliyon 2022, 8, e08893. [Google Scholar] [CrossRef]
- Caldas, A.R.; Catita, J.; Machado, R.; Ribeiro, A.; Cerqueira, F.; Horta, B.; Medeiros, R.; Lúcio, M.; Lopes, C.M. Omega-3- and Resveratrol-Loaded Lipid Nanosystems for Potential Use as Topical Formulations in Autoimmune, Inflammatory, and Cancerous Skin Diseases. Pharmaceutics 2021, 13, 1202. [Google Scholar] [CrossRef]
- Stamatas, G.N.; de Sterke, J.; Hauser, M.; von Stetten, O.; van der Pol, A. Lipid Uptake and Skin Occlusion Following Topical Application of Oils on Adult and Infant Skin. J. Dermatol. Sci. 2008, 50, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Mendes Aciole, I.H.; de Andrade Júnior, F.P.; Vilar Cordeiro, L.; Pereira de Souza, J.B. Aloe Gel: Manipulation and Characterization of Physical-Chemical Quality Adjustment. Rev. Colomb. Cienc. Quím.-Farm. 2020, 49, 790–805. [Google Scholar] [CrossRef]
- Borghetti, G.S.; Knorst, M.T. Desenvolvimento e avaliação da estabilidade física de loções O/A contendo filtros solares. Rev. Bras. Ciênc. Farm. 2006, 42, 531–537. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Kaleva, M.; Kroumov, A.; Slavkova, M.; Benbassat, N.; Yoncheva, K.; Najdenski, H. Advantageous Combinations of Nanoencapsulated Oregano Oil with Selected Antibiotics for Skin Treatment. Pharmaceutics 2022, 14, 2773. [Google Scholar] [CrossRef] [PubMed]











| Sample | F127 | NP-F127 | Bud-NP-F127 | MC | NP-MC | Bud-NP-MC |
|---|---|---|---|---|---|---|
| G′ (Pa) | 22,960 | 26,900 | 27,570 | 69 | 240 | 191 |
| G″ (Pa) | 3037 | 1847 | 1757 | 108 | 231 | 185 |
| η* (Pa.s) | 3686 | 4292 | 4396 | 20 | 52 | 49 |
| Formulation | Zero Order | First Order | Higuchi | Korsmeyer-Peppas |
|---|---|---|---|---|
| Bud-MC | R2 = 0.8870 | R2 = 0.9875 | R2 = 0.9874 | R2 = 0.8909 |
| k = 13.895 | k = −0.152 | k = 37.678 | n = 0.672 | |
| Bud-F127 | R2 = 0.8598 | R2 = 0.9848 | R2 = 0.9850 | R2 = 0.8344 |
| k = 14.567 | k = −0.425 | k = 40.073 | n = 0.613 | |
| Bud-NP | R2 = 0.871 | R2 = 0.9862 | R2 = 0.9874 | R2 = 0.8612 |
| k = 14.844 | k = −0.2176 | k = 40.619 | n = 0.413 | |
| Bud-NP-MC | R2 = 0.9673 | R2 = 0.9687 | R2 = 0.9604 | R2 = 0.9337 |
| k = 3.175 | k = −0.021 | k = 11.007 | n = 0.268 | |
| Bud-NP-F127 | R2 = 0.9599 | R2 = 0.8673 | R2 = 0.9089 | R2 = 0.9125 |
| k = 8.309 | k = −0.090 | k = 28.133 | n = 0.418 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavkova, M.; Lazov, C.; Spassova, I.; Kovacheva, D.; Tibi, I.P.-E.; Stefanova, D.; Tzankova, V.; Petrov, P.D.; Yoncheva, K. Formulation of Budesonide-Loaded Polymeric Nanoparticles into Hydrogels for Local Therapy of Atopic Dermatitis. Gels 2024, 10, 79. https://doi.org/10.3390/gels10010079
Slavkova M, Lazov C, Spassova I, Kovacheva D, Tibi IP-E, Stefanova D, Tzankova V, Petrov PD, Yoncheva K. Formulation of Budesonide-Loaded Polymeric Nanoparticles into Hydrogels for Local Therapy of Atopic Dermatitis. Gels. 2024; 10(1):79. https://doi.org/10.3390/gels10010079
Chicago/Turabian StyleSlavkova, Marta, Christophor Lazov, Ivanka Spassova, Daniela Kovacheva, Ivanka Pencheva-El Tibi, Denitsa Stefanova, Virginia Tzankova, Petar D. Petrov, and Krassimira Yoncheva. 2024. "Formulation of Budesonide-Loaded Polymeric Nanoparticles into Hydrogels for Local Therapy of Atopic Dermatitis" Gels 10, no. 1: 79. https://doi.org/10.3390/gels10010079