Impact of the Preparation Method on the Formulation Properties of Allantoin Hydrogels: Evaluation Using Semi-Solid Control Diagram (SSCD) Principles
Abstract
:1. Introduction
2. Results and Discussion
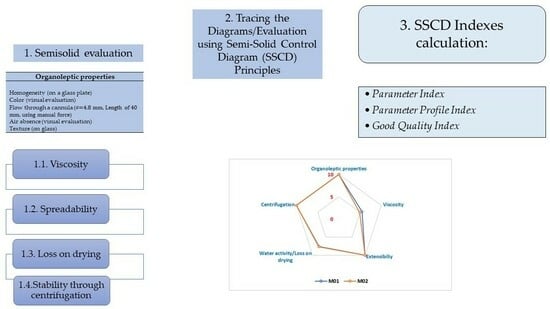
2.1. The Hydrogel Evaluation Using SSCDs
2.1.1. Organoleptic Properties
Homogeneity
Color
Flow through a Tube or Cannula
Air Absence
Texture
2.1.2. Viscosity
2.1.3. Extensibility (Spreadability)
2.1.4. Loss on Drying
2.1.5. Stability through Centrifugation
2.2. SSCDs Applied for the Hydrogels’ Evaluation
- Parameter Index (PI—Table 1). It was noticed that all the gels respected the minimum admitted limit of 0.5 regarding the PI, with the formulation obtaining values higher than 0.8 for all four hydrogels.
- Parameter Profile Index (PPI—Table 1). The PPI ranged between 7.27 for M2 and 8.6 for M01. It can be noticed that the M1, M01, and M02 presented values higher than 8 of these indexes, with the only gels that received a lower score of this index being M2. Even though the hydrogels can be differentiated using this index, all four hydrogels fulfilled the average limit of 5 proposed for this index.
- Good Quality Index (GQI—Table 1). The final and most important index calculated using this method is GQI, whose limit was set at 5. The values of GQI ranged between 5.45 (M3) and 6.45 (M01), all of them respecting the minimum admitted limit of 5. It was noticed that GQI was lower in comparison to PPI, a fact that can be explained by the low value of the reliability factor of 0.75.
2.3. Penetrometry (Consistency) Study
2.4. Rheology and Flow Behavior
2.5. Drug Content Analysis Using the UV-Vis Spectrophotometric Method
3. Conclusions
4. Materials and Methods
4.1. Blank and Allantoin Gel Preparation
4.2. The Evaluation of the Proposed Hydrogel Using SSCDs
- ○
- Parametric Index (PI).wherePI = nr of parameters > 5/number of total parameters (5)
- ○
- nr of parameters > 5—the number of parameters that are equal to or higher than 5; the limit of acceptance is 0.5, whilst the maximum value that can be obtained is 1.
- ○
- The Parametric Profile Index (PPI) represents the average value of the radius of all parameters.
- ○
- Good Quality Index (GQI) can be calculated using the formula:GQI = PPI × f
- ○
- f = reliability factor = polygon area/circle area, which was set at f = 0.75.
4.2.1. Organoleptic Properties
4.2.2. Viscosity/Rheology/Flow Behavior
4.2.3. Extensibility (Spreadability)
4.2.4. Loss on Drying
4.2.5. Stability through Centrifugation
4.3. Penetrometry (Consistency) Study
4.4. Drug Content Analysis Using the UV-Vis Spectrophotometric Method
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahsan, A.; Tian, W.-X.; Farooq, M.A.; Khan, D.H. An overview of hydrogels and their role in transdermal drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 574–584. [Google Scholar] [CrossRef]
- Don, T.-M.; Huang, M.-L.; Chiu, A.-C.; Kuo, K.-H.; Chiu, W.-Y.; Chiu, L.-H. Preparation of thermo-responsive acrylic hydrogels useful for the application in transdermal drug delivery systems. Mater. Chem. Phys. 2008, 107, 266–273. [Google Scholar] [CrossRef]
- Jung, H.; Kim, M.K.; Lee, J.Y.; Choi, S.W.; Kim, J. Adhesive hydrogel patch with enhanced strength and adhesiveness to skin for transdermal drug delivery. Adv. Funct. Mater. 2020, 30, 2004407. [Google Scholar] [CrossRef]
- Singh, P.; Carrier, A.; Chen, Y.; Lin, S.; Wang, J.; Cui, S.; Zhang, X. Polymeric microneedles for controlled transdermal drug delivery. J. Control. Release 2019, 315, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Moran, C.A.; Zavgorodnya, O.; Penman, A.D.; Kharlampieva, E.; Bridges, S.L., Jr.; Hergenrother, R.W.; Singh, J.A.; Wick, T.M. Development of gellan gum containing formulations for transdermal drug delivery: Component evaluation and controlled drug release using temperature responsive nanogels. Int. J. Pharm. 2016, 509, 465–476. [Google Scholar] [CrossRef]
- Mazzitelli, S.; Pagano, C.; Giusepponi, D.; Nastruzzi, C.; Perioli, L. Hydrogel blends with adjustable properties as patches for transdermal delivery. Int. J. Pharm. 2013, 454, 47–57. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-based hydrogels as sustained drug-delivery systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Amatya, R.; Kim, D.; Min, K.A.; Shin, M.C. Iron oxide nanoparticles-loaded hydrogels for effective topical photothermal treatment of skin cancer. J. Pharm. Investig. 2022, 52, 775–785. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S.; Pal, K.; Banerjee, I.; Pal, D. Gum-based hydrogels in drug delivery. In Biopolymer-Based Formulations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 605–645. [Google Scholar]
- Singh, B.; Sharma, K.; Dutt, S. Dietary fiber tragacanth gum based hydrogels for use in drug delivery applications. Bioact. Carbohydr. Diet. Fibre 2020, 21, 100208. [Google Scholar] [CrossRef]
- Jadav, M.; Pooja, D.; Adams, D.J.; Kulhari, H. Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents. Pharmaceutics 2023, 15, 402. [Google Scholar] [CrossRef]
- Pettitt, D.J. Xanthan Gum. In Food hydrocolloids; CRC Press: Boca Raton, FL, USA, 2020; pp. 127–149. [Google Scholar]
- Raschip, I.E.; Vasile, C.; Ciolacu, D.; Cazacu, G. Semi-interpenetrating polymer networks containing polysaccharides. I Xanthan Lignin Netw. High Perform. Polym. 2007, 19, 603–620. [Google Scholar] [CrossRef]
- Bhunia, T.; Giri, A.; Nasim, T.; Chattopadhyay, D.; Bandyopadhyay, A. Uniquely different PVA-xanthan gum irradiated membranes as transdermal diltiazem delivery device. Carbohydr. Polym. 2013, 95, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Dvořák, J.; Rosiak, N.; Tykarska, E.; Szymańska, E.; Winnicka, K.; Ruchała, M.A.; Cielecka-Piontek, J. Buccal resveratrol delivery system as a potential new concept for the periodontitis treatment. Pharmaceutics 2021, 13, 417. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Villa, M.; Nácher, A.; Hernández, M.J.; Busó, M.O.V.; Barrachina, M.; Peñalver, N.; Díez-Sales, O. A novel lidocaine hydrochloride mucoadhesive films for periodontal diseases. J. Mater. Sci. Mater. Med. 2019, 30, 14. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Rachamalla, S.S.; Sistla, R. Xanthan gum stabilized gold nanoparticles: Characterization, biocompatibility, stability and cytotoxicity. Carbohydr. Polym. 2014, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, Q.; Tao, Y.; Wang, X. Rheological and pH dependent properties of injectable and controlled release hydrogels based on mushroom hyperbranched polysaccharide and xanthan gum. Carbohydr. Polym. Technol. Appl. 2021, 2, 100063. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Azambuja, J.H.; da Silveira, E.F.; Cervi, V.F.; Marchiori, M.C.L.; Maria-Engler, S.S.; Wink, M.R.; Azevedo, J.G.; Nogueira, C.W. Xanthan gum-based hydrogel containing nanocapsules for cutaneous diphenyl diselenide delivery in melanoma therapy. Investig. New Drugs 2020, 38, 662–674. [Google Scholar] [CrossRef]
- Lee, M.H.; Shin, G.H.; Park, H.J. Solid lipid nanoparticles loaded thermoresponsive pluronic–xanthan gum hydrogel as a transdermal delivery system. J. Appl. Polym. Sci. 2018, 135, 46004. [Google Scholar] [CrossRef]
- Aman, R.M.; Zaghloul, R.A.; El-Dahhan, M.S. Formulation, optimization and characterization of allantoin-loaded chitosan nanoparticles to alleviate ethanol-induced gastric ulcer: In-vitro and in-vivo studies. Sci. Rep. 2021, 11, 2216. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 204, Allantoin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Allantoin (accessed on 23 May 2023).
- Thornfeldt, C. Cosmeceuticals containing herbs: Fact, fiction, and future. Dermatol. Surg. 2005, 31, 873–881. [Google Scholar] [CrossRef]
- Bakibaev, A.; Il’Yasov, S.; Tatarenko, O.; Tuguldurova, V.; Zorin, A.; Malkov, V.; Kasyanova, A. Allantoin: Synthesis and chemical properties. Bull. Karaganda Univ. Ser. Chem. 2020, 97, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Acuña, R.A.; Meza-Valle, K.Z.; Cuevas-González, J.C.; Ordoñez-Casanova, E.G.; Castellanos-García, M.I.; Zaragoza-Contreras, E.A.; Tamayo-Pérez, G.F. Characterization and In Vivo Assay of Allantoin-Enriched Pectin Hydrogel for the Treatment of Skin Wounds. Int. J. Mol. Sci. 2023, 24, 7377. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Matricardi, P.; Cencetti, C.; Peris, J.E.; Melis, V.; Carbone, C.; Escribano, E.; Zaru, M.; Fadda, A.M.; Manconi, M. Combination of argan oil and phospholipids for the development of an effective liposome-like formulation able to improve skin hydration and allantoin dermal delivery. Int. J. Pharm. 2016, 505, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Nardi-Ricart, A.; Linares, M.J.; Villca-Pozo, F.; Pérez-Lozano, P.; Suñé-Negre, J.M.; Bachs-deMiquel, L.; Roig-Carreras, M.; Suñé-Pou, M.; Nofrerias-Roig, I.; García-Montoya, E. A new design for the review and appraisal of semi-solid dosage forms: Semi-solid Control Diagram (SSCD). PLoS ONE 2018, 13, e0201643. [Google Scholar] [CrossRef] [PubMed]
- Pintea, A.; Vlad, R.-A.; Antonoaea, P.; Rédai, E.M.; Todoran, N.; Barabás, E.-C.; Ciurba, A. Structural Characterization and Optimization of a Miconazole Oral Gel. Polymers 2022, 14, 5011. [Google Scholar] [CrossRef]
- Mut, A.M.; Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.; Stănciulescu, C.; Mitu, M.A.; Szabadai, Z.; Lupuleasa, D. Chitosan/HPMC based hydrogels containing essential oils for topical delivery of fluconazole: Preliminary studies. Farmacia 2018, 66, 248–256. [Google Scholar]
- Karampelas, O.; Balaci, T.D.; Funieru, C.; Ozon, E.A.; Mitu, M.A.; Fița, C.A.; Lupuliasa, D. Rheological behaviour and in vitro release profiles of naftifine topical hydrogels. Farmacia 2020, 68, 1120–1128. [Google Scholar] [CrossRef]
- Uniformity of Dosage Units. European Pharmacopoeia, 11th ed.; European Directorate for the Quality of Medicines: Strasburg, France, 2023.
- Gaikwad, S.S.; Kothule, A.M.; Morade, Y.Y.; Patil, S.S.; Laddha, U.D.; Kshirsagar, S.J.; Salunkhe, K.S. An overview of the implementation of SeDeM and SSCD in various formulation developments. Int. J. Pharm. 2023, 25, 122699. [Google Scholar] [CrossRef]
- Vlad, R.-A.; Antonoaea, P.; Todoran, N.; Muntean, D.-L.; Redai, E.-M.; Silasi, O.-A.; Tătaru, A.; Bîrsan, M.; Imre, S.; Ciurba, A. Pharmacotechnical and analytical preformulation studies for cannabidiol orodispersible tablets. Saudi Pharm. J. 2021, 29, 1029–1042. [Google Scholar] [CrossRef]
- Ciurba, A.; Popescu, A.G.; Vlad, R.-A.; Redai, E.; Todoran, N.; Cotoi, C.T.; Bîrsan, M.; Antonoaea, P. Development and evaluation of bigels containing naproxen sodium for topical administration. Farmacia 2023, 71, 771–780. [Google Scholar] [CrossRef]








| Index | M1 | M2 | M01 | M02 |
|---|---|---|---|---|
| PI | 1 | 0.8 | 1 | 0.8 |
| PPI | 8.36 | 7.27 | 8.6 | 8.5 |
| GQI | 6.27 | 5.45 | 6.45 | 6.38 |
| GQIa | 8.36 | 7.26 | 8.6 | 8.51 |
| Ingredient | Role | Formulation Code | |||
|---|---|---|---|---|---|
| M01 | M02 | M1 | M2 | ||
| Allantoin | Active ingredient | - | - | 1 | 1 |
| Xanthan gum | Gel-forming agent | 1 | 1 | 1 | 1 |
| Citric acid | Keratin softener | 1 | 1 | 1 | 1 |
| Glycerol | Humectant | 5 | 5 | 5 | 5 |
| Preservative solution | Preservative/Dispersion media | ad 100 | ad 100 | ad 100 | ad 100 |
| Parameters/Test | Limit Values | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Homogeneity (on a glass plate) | Discontinuities that can be noticed visually (inhomogeneous dispersion) | Small discontinuities can be noticed with a microscope (partially homogenous) | No physical discontinuities can be noticed (homogenous) |
| Color (visual evaluation) | Different shades can be noticed | Non-uniform parts are almost imperceptible | Uniform |
| Flow through a cannula (Ø = 4.8 mm; length of 40 mm, using manual force) | Excessive force is required for flowing | Flows with difficulty | Passes smoothly |
| Air absence (visual evaluation) | Air bubbles are observed by visual evaluation | Air bubbles can be noticed with the help of a microscope | Air absence through microscope visualization |
| Texture (on glass) | Difficult to spread | - | It can spread properly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlad, R.-A.; Dudici, T.-C.; Syed, M.A.; Antonoaea, P.; Rédai, E.M.; Todoran, N.; Cotoi, C.-T.; Bîrsan, M.; Ciurba, A. Impact of the Preparation Method on the Formulation Properties of Allantoin Hydrogels: Evaluation Using Semi-Solid Control Diagram (SSCD) Principles. Gels 2024, 10, 58. https://doi.org/10.3390/gels10010058
Vlad R-A, Dudici T-C, Syed MA, Antonoaea P, Rédai EM, Todoran N, Cotoi C-T, Bîrsan M, Ciurba A. Impact of the Preparation Method on the Formulation Properties of Allantoin Hydrogels: Evaluation Using Semi-Solid Control Diagram (SSCD) Principles. Gels. 2024; 10(1):58. https://doi.org/10.3390/gels10010058
Chicago/Turabian StyleVlad, Robert-Alexandru, Teodora-Cătălina Dudici (Vlăgea), Muhammad Ali Syed, Paula Antonoaea, Emöke Margit Rédai, Nicoleta Todoran, Cornelia-Titiana Cotoi, Magdalena Bîrsan, and Adriana Ciurba. 2024. "Impact of the Preparation Method on the Formulation Properties of Allantoin Hydrogels: Evaluation Using Semi-Solid Control Diagram (SSCD) Principles" Gels 10, no. 1: 58. https://doi.org/10.3390/gels10010058