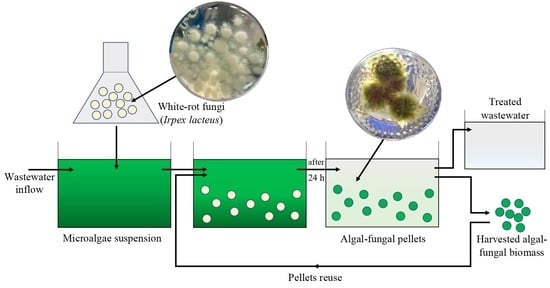
Microalgae Harvesting after Tertiary Wastewater Treatment with White-Rot Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Wastewater Source
2.3. Experimental Setup
2.4. Experimental Harvesting Conditions
2.5. Microalgal Cell Measurements
2.6. Statistical Analysis
3. Results & Discussion
3.1. Microalgae Harvesting with Various Fungal Species
3.2. Effect of the Reuse of Algal-Fungal Pellets
3.3. Impact of Bio-Flocculation Conditions
3.4. Algal-Fungal Co-Cultivation in Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Aliyu, A.; Lee, J.G.M.; Harvey, A.P. Microalgae for biofuels: A review of thermochemical conversion processes and associated opportunities and challenges. Bioresour. Technol. Rep. 2021, 15, 100694. [Google Scholar] [CrossRef]
- Ali, S.; Paul Peter, A.; Chew, K.W.; Munawaroh, H.; Show, P.L. Resource recovery from industrial effluents through the cultivation of microalgae: A review. Bioresour. Technol. 2021, 337, 125461. [Google Scholar] [CrossRef] [PubMed]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.C.; Tian, Y.; Liang, H.; Zuo, W.; Wang, Z.W.; Zhang, J.; He, Z.W. Enhanced nitrogen and phosphorus removal from domestic wastewater via algae-assisted sequencing batch biofilm reactor. Bioresour. Technol. 2018, 250, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Shahid, M.K.; Dash, R.R.; Bhunia, P.; Liu, D.; Varjani, S.; Zhang, T.C.; Surampalli, R.Y. Nutrient removal from domestic wastewater: A comprehensive review on conventional and advanced technologies. J. Environ. Manag. 2021, 296, 113246. [Google Scholar] [CrossRef]
- Tiwari, A.; Kiran, T.; Pandey, A. Chapter 14-Algal cultivation for biofuel production. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 383–403. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Chu, R.; Li, S.; Zhu, L.; Yin, Z.; Hu, D.; Liu, C.; Mo, F. A review on co-cultivation of microalgae with filamentous fungi: Efficient harvesting, wastewater treatment and biofuel production. Renew. Sustain. Energy Rev. 2021, 139, 110689. [Google Scholar] [CrossRef]
- Smith, B.; Davis, R. Sedimentation of algae flocculated using naturally-available, magnesium-based flocculants. Algal Res. 2012, 1, 32–39. [Google Scholar] [CrossRef]
- Papazi, A.; Makridis, P.; Divanach, P. Harvesting Chlorella minutissima using cell coagulants. J. Appl. Phycol. 2010, 22, 349–355. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.L.; Smith, R.W. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Barros, A.; Gonçalves, A.L.; Simões, M.; Pires, J.C. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Najjar, Y.S.H.; Abu-Shamleh, A. Harvesting of microalgae by centrifugation for biodiesel production: A review. Algal Res. 2020, 51, 02046. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Sommerfeld, M.; Puruhito, E.; Chen, Y. Harvesting algal biomass for biofuels using ultrafiltration membranes. Bioresour. Technol. 2010, 101, 5297–5304. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae-An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, G.; Sun, S.; Hu, C.; Liu, J. Co-pelletization of microalgae and fungi for efficient nutrient purification and biogas upgrading. Bioresour. Technol. 2019, 289, 121656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, B. A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Bioresour. Technol. 2012, 114, 529–535. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, L.; Li, P.; Yan, Y.; Chen, T.; Zheng, T.; Wang, H. Flocculation mechanism of Aspergillus niger on harvesting of Chlorella vulgaris biomass. Algal Res. 2017, 25, 402–412. [Google Scholar] [CrossRef]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PLoS ONE 2014, 9, e113497. [Google Scholar] [CrossRef] [Green Version]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-assisted algal flocculation: Application in wastewater treatment and biofuel production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Min, M.; Hu, B.; Ma, X.; Liu, Y.; Wang, Q.; Shi, J.; Chen, P.; Ruan, R. Filamentous fungi assisted bio-flocculation: A novel alternative technique for harvesting heterotrophic and autotrophic microalgal cells. Sep. Purif. Technol. 2013, 107, 158–165. [Google Scholar] [CrossRef]
- Alrubaie, G.; Al-Shammari, R.H.H. Microalgae Chlorella Vulgaris Harvesting Via Co-Pelletization with Filamentous Fungus. Baghdad Sci. J. 2018, 15, 31–36. [Google Scholar] [CrossRef]
- Al-Hothaly, K.A.; Adetutu, E.M.; Taha, M.; Fabbri, D.; Lorenzetti, C.; Conti, R.; May, B.H.; Shar, S.S.; Bayoumi, R.A.; Ball, A.S. Bio-harvesting and pyrolysis of the microalgae Botryococcus braunii. Bioresour. Technol. 2015, 191, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Li, W.; Chen, J.; Leng, S.; Chen, J.; Wei, L.; Peng, H.; Li, J.; Zhou, W.; Huang, H. Co-culture of fungi-microalgae consortium for wastewater treatment: A review. Bioresour. Technol. 2021, 330, 125008. [Google Scholar] [CrossRef] [PubMed]
- Dalecka, B.; Juhna, T.; Rajarao, G.K. Constructive use of filamentous fungi to remove pharmaceutical substances from wastewater. J. Water Process Eng. 2020, 33, 100992. [Google Scholar] [CrossRef]
- Mezule, L.; Civzele, A. Bioprospecting White-Rot Basidiomycete Irpex lacteus for Improved Extraction of Lignocellulose-Degrading Enzymes and Their Further Application. J. Fungi 2020, 6, 256. [Google Scholar] [CrossRef]
- Mezule, L.; Berzina, I.; Strods, M. The Impact of Substrate–Enzyme Proportion for Efficient Hydrolysis of Hay. Energies 2019, 12, 3526. [Google Scholar] [CrossRef] [Green Version]
- Laezza, C.; Salbitani, G.; Carfagna, S. Fungal Contamination in Microalgal Cultivation: Biological and Biotechnological Aspects of Fungi-Microalgae Interaction. J. Fungi 2022, 8, 1099. [Google Scholar] [CrossRef]
- Dagenais, T.R.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [Green Version]
- Paulussen, C.; Hallsworth, J.E.; Álvarez-Pérez, S.; Nierman, W.C.; Hamill, P.G.; Blain, D.; Rediers, H.; Lievens, B. Ecology of aspergillosis: Insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb. Biotechnol. 2017, 10, 296–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.; Wannemuehler, K.A.; Marr, K.A.; Hadley, S.; Kontoyiannis, D.P.; Walsh, T.J.; Fridkin, S.K.; Pappas, P.G.; Warnock, D.W. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: Interim results of a prospective multicenter surveillance program. Med. Mycol. 2005, 43 (Suppl. 1), S49–S58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 5815-1:2019; Water Quality—Determination of Biochemical Oxygen Demand after n Days (BODn)—Part 1: Dilution and Seeding Method with Allylthiourea Addition. International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/69058.html (accessed on 2 November 2022).
- ISO 6060:1989; Water Quality—Determination of the Chemical Oxygen Demand. International Organization for Standardization: Geneva, Switzerland, 1989. Available online: https://www.iso.org/standard/12260.html (accessed on 2 November 2022).
- BS EN 872:2005; Water Quality. Determination of Suspended Solids. Method by Filtration through Glass Fibre Filters. European Standards: Pilsen, Czech Republic, 2005. Available online: https://www.en-standard.eu/bs-en-872-2005-water-quality-determination-of-suspended-solids-method-by-filtration-through-glass-fibre-filters/ (accessed on 2 November 2022).
- ISO 7150-1:1984; Water Quality—Determination of Ammonium—Part 1: Manual Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 1984. Available online: https://www.iso.org/standard/13742.html (accessed on 2 November 2022).
- ISO 6777:1984; Water Quality—Water Quality—Determination of Nitrite—Molecular Absorption Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 1984. Available online: https://www.iso.org/standard/13273.html (accessed on 2 November 2022).
- ISO 7890-3:1988; Water Quality—Determination of Nitrate—Part 3: Spectrometric Method Using Sulfosalicylic Acid. International Organization for Standardization: Geneva, Switzerland, 1988. Available online: https://www.iso.org/standard/14842.html (accessed on 2 November 2022).
- ISO 6878:2004; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 2004. Available online: https://www.iso.org/standard/36917.html (accessed on 2 November 2022).
- ISO 0523:2008; Water Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/51994.html (accessed on 2 November 2022).
- Lavrinovičs, A.; Mežule, L.; Juhna, T. Microalgae starvation for enhanced phosphorus uptake from municipal wastewater. Algal Res. 2020, 52. [Google Scholar] [CrossRef]
- Denisova, V.; Mezule, L.; Juhna, T. The effect of chitosan nanoparticles on Escherichia coli viability in drinking water disinfection. Water Pract. Technol. 2022, 17, 537–543. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K. Insights into lignin degradation and its potential industrial applications. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar] [CrossRef]
- Saraswat, P.; Yadav, K.; Gupta, A.; Prasad, M.; Ranjan, R. Chapter 34-Physiological and molecular basis for remediation of polyaromatic hydrocarbons. In Handbook of Bioremediation; Hasanuzzaman, M., Prasad, M.N.V., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 535–550. [Google Scholar] [CrossRef]
- Kalinoski, R.M. White-Rot Fungi as Pretreatment Agents for Wood Destined for Biofuel Applications. Masters Theses, Eastern Illinois University, Charleston, IL, USA, 2016; p. 2450. [Google Scholar]
- Canam, T.; Dumonceaux, T.J.; Record, E.; Li, Y. White-rot fungi: The key to sustainable biofuel production? Biofuels 2013, 4, 247–250. [Google Scholar] [CrossRef]
- Jurado, M.; Martinèz, À.T.; Martinez, M.J.; Saparrat, M.C.N. 6.45-Application of White-Rot Fungi in Transformation, Detoxification, or Revalorization of Agriculture Wastes: Role of Laccase in the Processes. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 595–603. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Huang, Z.; Guo, Z.; Huang, T.; Peng, M.; Shi, J.; Hu, L. Applications of white rot fungi in bioremediation with nanoparticles and biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 4853–4862. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef]
- Göçenoğlu, A.; Pazarlioglu, N. Cinnabarinic acid: Enhanced production from Pycnoporus cinnabarinus, characterization, structural and functional properties. Hacet. J. Biol. Chem. 2014, 42, 281–290. [Google Scholar] [CrossRef]
- Nasir, N.M.; Bakar, N.S.; Lananan, F.; Abdul Hamid, S.H.; Lam, S.S.; Jusoh, A. Treatment of African catfish, Clarias gariepinus wastewater utilizing phytoremediation of microalgae, Chlorella sp. with Aspergillus niger bio-harvesting. Bioresour. Technol. 2015, 190, 492–498. [Google Scholar] [CrossRef]
- Oliveira, H.R.; Bassin, I.D.; Cammarota, M.C. Bioflocculation of cyanobacteria with pellets of Aspergillus niger: Effects of carbon supplementation, pellet diameter, and other factors in biomass densification. Bioresour. Technol. 2019, 294, 122167. [Google Scholar] [CrossRef] [PubMed]
- Tejido-Nuñez, Y.; Aymerich, E.; Sancho, L.; Refardt, D. Treatment of aquaculture effluent with Chlorella vulgaris and Tetradesmus obliquus: The effect of pretreatment on microalgae growth and nutrient removal efficiency. Ecol. Eng. 2019, 136, 1–9. [Google Scholar] [CrossRef]
- Rugnini, L.; Ellwood, N.; Costa, G.; Falsetti, A.; Congestri, R.; Bruno, L. Scaling-up of wastewater bioremediation by Tetradesmus obliquus, sequential bio-treatments of nutrients and metals. Ecotoxicol. Environ. Saf. 2019, 172, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Rugnini, L.; Costa, G.; Congestri, R.; Antonaroli, S.; Sanità di Toppi, L.; Bruno, L. Phosphorus and metal removal combined with lipid production by the green microalga Desmodesmus sp.: An integrated approach. Plant Physiol. Biochem. 2018, 125, 45–51. [Google Scholar] [CrossRef]
- Kong, W.; Kong, J.; Ma, J.; Lyu, H.; Feng, S.; Wang, Z.; Yuan, P.; Shen, B. Chlorella vulgaris cultivation in simulated wastewater for the biomass production, nutrients removal and CO2 fixation simultaneously. J. Environ. Manag. 2021, 284, 112070. [Google Scholar] [CrossRef]
- Al hattab, M.; Ghaly, A.; Hammouda, A. Microalgae Harvesting Methods for Industrial Production of Biodiesel: Critical Review and Comparative Analysis. J. Fundam Renew. Energy Appl. 2015, 5, 154. [Google Scholar] [CrossRef]







| Parameter | Method | Primary Wastewater | Secondary Wastewater | |
|---|---|---|---|---|
| BOD5 (biochemical oxygen demand) | ISO 5815-1 | 160 | 6 | mg/L |
| COD (chemical oxygen demand) | ISO 6060 | 480 | 39 | mg/L |
| SS (suspended solids) | EN 872 | 210 | 6 | mg/L |
| NH4-N (dissolved ammonium) | ISO 7150-1 | 45 | 2.02 | mg/L |
| NO2-N (dissolved nitrite) | ISO 6777:1984 + AC:2001 | 0.054 | mg/L | |
| NO3-N (dissolved nitrate) | ISO 7890-3 | 3.22 | mg/L | |
| PO4-P (dissolved phosphate) | ISO 6878 | 3.9 | 0.29 | mg/L |
| pH | ISO 10523 | 6.9 | 7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Civzele, A.; Mezule, L. Microalgae Harvesting after Tertiary Wastewater Treatment with White-Rot Fungi. J. Fungi 2022, 8, 1232. https://doi.org/10.3390/jof8111232
Civzele A, Mezule L. Microalgae Harvesting after Tertiary Wastewater Treatment with White-Rot Fungi. Journal of Fungi. 2022; 8(11):1232. https://doi.org/10.3390/jof8111232
Chicago/Turabian StyleCivzele, Anna, and Linda Mezule. 2022. "Microalgae Harvesting after Tertiary Wastewater Treatment with White-Rot Fungi" Journal of Fungi 8, no. 11: 1232. https://doi.org/10.3390/jof8111232