Investigating Candida glabrata Urinary Tract Infections (UTIs) in Mice Using Bioluminescence Imaging
Abstract
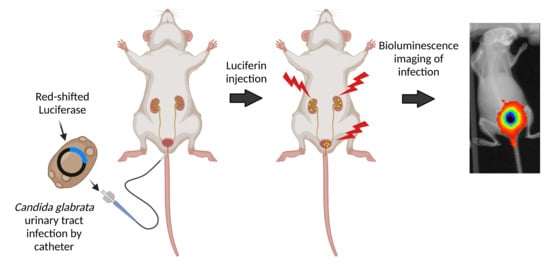
:1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Growth Media
2.2. Urinary Tract Infection in Mice
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. In Vitro Luminescence Measurement
2.5. In Vivo Bioluminescence and Antifungal Treatment
3. Results
3.1. Fate of C. glabrata during Urinary Tract Infections
3.2. Expression of a Codon Adapted Red-Shifted Luciferase from the ENO1-Promoter Results in High Bioluminescence In Vitro
3.3. Expression of a Codon Adapted Red-Shifted Luciferase from the TEF1-Promoter Results in the Highest Luminescence In Vivo
3.4. In Vivo Monitoring of Treatment of a C. glabrata Urinary Tract Infection with Fluconazole
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef] [Green Version]
- Bolotin-Fukuhara, M.; Fairhead, C. Candida glabrata: A deadly companion? Yeast 2014, 31, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Maccallum, D.M. Hosting infection: Experimental models to assay Candida virulence. Int. J. Microbiol. 2012, 2012, 363764. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty years of the SENTRY antifungal surveillance program: Results for Candida species from 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [Green Version]
- Horn, D.L.; Neofytos, D.; Anaissie, E.J.; Fishman, J.A.; Steinbach, W.J.; Olyaei, A.J.; Marr, K.A.; Pfaller, M.A.; Chang, C.H.; Webster, K.M. Epidemiology and outcomes of candidemia in 2019 patients: Data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 2009, 48, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Gabaldon, T.; Martin, T.; Marcet-Houben, M.; Durrens, P.; Bolotin-Fukuhara, M.; Lespinet, O.; Arnaise, S.; Boisnard, S.; Aguileta, G.; Atanasova, R.; et al. Comparative genomics of emerging pathogens in the Candida glabrata clade. BMC Genom. 2013, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Robnett, C.J. Three new insect-associated species of the yeast genus Candida. Can. J. Microbiol. 1998, 44, 965–973. [Google Scholar] [CrossRef]
- Roetzer, A.; Gabaldon, T.; Schuller, C. From Saccharomyces cerevisiae to Candida glabratain a few easy steps: Important adaptations for an opportunistic pathogen. FEMS Microbiol. Lett. 2011, 314, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtzman, C.P.; Robnett, C.J. Phylogenetic relationships among yeasts of the ‘Saccharomyces complex’ determined from multigene sequence analyses. FEMS Yeast Res. 2003, 3, 417–432. [Google Scholar] [CrossRef] [Green Version]
- Domergue, R.; Castano, I.; De Las Penas, A.; Zupancic, M.; Lockatell, V.; Hebel, J.R.; Johnson, D.; Cormack, B.P. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science 2005, 308, 866–870. [Google Scholar] [CrossRef]
- Brieland, J.; Essig, D.; Jackson, C.; Frank, D.; Loebenberg, D.; Menzel, F.; Arnold, B.; DiDomenico, B.; Hare, R. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect. Immun. 2001, 69, 5046–5055. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.; Horn, T.; Frimodt-Moller, N. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 2002, 30, 286–291. [Google Scholar] [CrossRef]
- Ferrari, S.; Sanguinetti, M.; De Bernardis, F.; Torelli, R.; Posteraro, B.; Vandeputte, P.; Sanglard, D. Loss of mitochondrial functions associated with azole resistance in Candida glabrata results in enhanced virulence in mice. Antimicrob. Agents Chemother. 2011, 55, 1852–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, S.; Sanguinetti, M.; Torelli, R.; Posteraro, B.; Sanglard, D. Contribution of CgPDR1-regulated genes in enhanced virulence of azole-resistant Candida glabrata. PLoS ONE 2011, 6, e17589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, H.; Ueno, K.; Uno, J.; Nagi, M.; Tanabe, K.; Aoyama, T.; Chibana, H.; Bard, M. Growth defects resulting from inhibiting ERG20 and RAM2 in Candida glabrata. FEMS Microbiol. Lett. 2011, 317, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Van Dyck, K.; Rogiers, O.; Vande Velde, G.; Van Dijck, P. Let’s shine a light on fungal infections: A noninvasive imaging toolbox. PLoS Pathog. 2020, 16, e1008257. [Google Scholar] [CrossRef]
- Dorsaz, S.; Coste, A.T.; Sanglard, D. Red-shifted firefly luciferase optimized for Candida albiicans in vivo bioluminescence imaging. Front. Microbiol. 2017, 8, 1478. [Google Scholar] [CrossRef] [PubMed]
- Vande Velde, G.; Kucharikova, S.; Van Dijck, P.; Himmelreich, U. Bioluminescence imaging of fungal biofilm development in live animals. Methods Mol. Biol. 2014, 1098, 153–167. [Google Scholar] [CrossRef]
- Mosci, P.; Pericolini, E.; Gabrielli, E.; Kenno, S.; Perito, S.; Bistoni, F.; d’Enfert, C.; Vecchiarelli, A. A novel bioluminescence mouse model for monitoring oropharyngeal candidiasis in mice. Virulence 2013, 4, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Enjalbert, B.; Rachini, A.; Vediyappan, G.; Pietrella, D.; Spaccapelo, R.; Vecchiarelli, A.; Brown, A.J.; d’Enfert, C. A multifunctional, synthetic Gaussia princeps luciferase reporter for live imaging of Candida albicans infections. Infect. Immun. 2009, 77, 4847–4858. [Google Scholar] [CrossRef] [Green Version]
- Pietrella, D.; Enjalbert, B.; Zeidler, U.; Znaidi, S.; Rachini, A.; Vecchiarelli, A.; d’Enfert, C. A luciferase reporter for gene expression studies and dynamic imaging of superficial Candida albicans infections. Methods Mol. Biol. 2012, 845, 537–546. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Bremer, C.; Weissleder, R. Fluorescence imaging with near-infrared light: New technological advances that enable in vivo molecular imaging. Eur. Radiol. 2003, 13, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vande Velde, G.; Kucharikova, S.; Van Dijck, P.; Himmelreich, U. Bioluminescence imaging increases in vivo screening efficiency for antifungal activity against device-associated Candida albicans biofilms. Int. J. Antimicrob. Agents 2018, 52, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Persyn, A.; Rogiers, O.; Brock, M.; Vande Velde, G.; Lamkanfi, M.; Jacobsen, I.D.; Himmelreich, U.; Lagrou, K.; Van Dijck, P.; Kucharikova, S. Monitoring of fluconazole and caspofungin activity against in vivo Candida glabrata biofilms by bioluminescence imaging. Antimicrob. Agents Chemother. 2019, 63, e01555-18. [Google Scholar] [CrossRef] [Green Version]
- Van Dyck, K.; Van Dijck, P.; Vande Velde, G. Bioluminescence imaging to study mature biofilm formation by Candida spp. and antifungal activity in vitro and in vivo. Methods Mol. Biol. 2020, 2081, 127–143. [Google Scholar] [CrossRef]
- Kaur, R.; Domergue, R.; Zupancic, M.L.; Cormack, B.P. A yeast by any other name: Candida glabrata and its interaction with the host. Curr. Opin. Microbiol. 2005, 8, 378–384. [Google Scholar] [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.; Azeredo, J. Silicone colonization by non-Candida albicans Candida species in the presence of urine. J. Med. Microbiol. 2010, 59, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Gharaghani, M.; Taghipour, S.; Halvaeezadeh, M.; Mahmoudabadi, A.Z. Candiduria: A review article with specific data from Iran. Turk. J. Urol. 2018, 44, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Diaz, E.; Hernandez-Rios, C.J.; Bravo-Cuellar, A. Antibiotic resistance: Microbiological profile of urinary tract infections in Mexico. Cir. Cir. 2019, 87, 176–182. [Google Scholar] [CrossRef]
- Peng, D.; Li, X.; Liu, P.; Luo, M.; Chen, S.; Su, K.; Zhang, Z.; He, Q.; Qiu, J.; Li, Y. Epidemiology of pathogens and antimicrobial resistanceof catheter-associated urinary tract infections in intensivecare units: A systematic review and meta-analysis. Am. J. Infect. Control 2018, 46, e81–e90. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.M.P.; Hoffmann-Santos, H.D.; Dias, L.B.; Tadano, T.; Karhawi, A.S.K.; Dutra, V.; Candido, S.L.; Hahn, R.C. Epidemiological profile of patients hospitalized with candiduria in the central-western region of Brazil. Rev. Iberoam. Micol. 2019, 36, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Misra, V.; Ranjan, N.; Jain, S.B.; Gandhi, S. Speciation, biofilm formation and antifungal susceptibility of Candida isolates from clinically diagnosed patient of UTI in a tertiary care hospital. J. Assoc. Physicians India 2019, 67, 42–45. [Google Scholar] [PubMed]
- Gajdacs, M.; Doczi, I.; Abrok, M.; Lazar, A.; Burian, K. Epidemiology of candiduria and Candida urinary tract infections in inpatients and outpatients: Results from a 10-year retrospective survey. Cent. Eur. J. Urol. 2019, 72, 209–214. [Google Scholar] [CrossRef]
- Denis, B.; Chopin, D.; Piron, P.; Resche-Rigon, M.; Bretagne, S.; Gits-Muselli, M.; Peraldi, M.N.; Abboud, I.; Molina, J.M. Candiduria in kidney transplant recipients: Is antifungal therapy useful? Mycoses 2018, 61, 298–304. [Google Scholar] [CrossRef]
- Vale-Silva, L.; Ischer, F.; Leibundgut-Landmann, S.; Sanglard, D. Gain-of-function mutations in PDR1, a regulator of antifungal drug resistance in Candida glabrata, control adherence to host cells. Infect. Immun. 2013, 81, 1709–1720. [Google Scholar] [CrossRef] [Green Version]
- Zordan, R.E.; Ren, Y.; Pan, S.-J.; Rotondo, G.; de Las Peñas, A.; Iluore, J.; Cormack, B.P. Expression plasmids for use in Candida glabrata. G3 Genes Genomes Genet. 2013, 3, 1675–1686. [Google Scholar] [CrossRef] [Green Version]
- Vale-Silva, L.; Beaudoing, E.; Tran, V.D.T.; Sanglard, D. Comparative genomics of two sequential Candida glabrata clinical isolates. G3 Genes Genomes Genet. 2017, 7, 2413–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vale-Silva, L.A.; Moeckli, B.; Torelli, R.; Posteraro, B.; Sanguinetti, M.; Sanglard, D. Upregulation of the adhesin gene EPA1 mediated by PDR1 in Candida glabrata leads to enhanced host colonization. mSphere 2016, 1, e00065-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.; Henriques, M.; Hayes, A.; Oliveira, R.; Azeredo, J.; Williams, D.W. Candida glabrata and Candida albicans co-infection of an in vitro oral epithelium. J. Oral Pathol. Med. 2011, 40, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Perez-Torrado, R.; Querol, A. Saccharomyces cerevisiae show low levels of traversal across human endothelial barrier in vitro. F1000Research 2017, 6, 944. [Google Scholar] [CrossRef]
- Galocha, M.; Pais, P.; Cavalheiro, M.; Pereira, D.; Viana, R.; Teixeira, M.C. Divergent approaches to virulence in C. albicans and C. glabrata: Two sides of the same coin. Int. J. Mol. Sci. 2019, 20, 2345. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, Y.; Tsai, H.F.; Myers, T.G.; Bennett, J.E. Transcriptional profiling of Candida glabrata during phagocytosis by neutrophils and in the infected mouse spleen. Infect. Immun. 2013, 81, 1325–1333. [Google Scholar] [CrossRef] [Green Version]
- Duggan, S.; Essig, F.; Hunniger, K.; Mokhtari, Z.; Bauer, L.; Lehnert, T.; Brandes, S.; Hader, A.; Jacobsen, I.D.; Martin, R.; et al. Neutrophil activation by Candida glabrata but not Candida albicans promotes fungal uptake by monocytes. Cell Microbiol. 2015, 17, 1259–1276. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Majer, O.; Frohner, I.E.; Lesiak-Markowicz, I.; Hildering, K.S.; Glaser, W.; Stockinger, S.; Decker, T.; Akira, S.; Muller, M.; et al. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-beta signaling. J. Immunol. 2011, 186, 3104–3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedelberger, M.; Penninger, P.; Tscherner, M.; Seifert, M.; Jenull, S.; Brunnhofer, C.; Scheidl, B.; Tsymala, I.; Bourgeois, C.; Petryshyn, A.; et al. Type I interferon response dysregulates host iron homeostasis and enhances Candida glabrata infection. Cell Host Microbe. 2020, 27, 454–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seider, K.; Brunke, S.; Schild, L.; Jablonowski, N.; Wilson, D.; Majer, O.; Barz, D.; Haas, A.; Kuchler, K.; Schaller, M.; et al. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J. Immunol. 2011, 187, 3072–3086. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.N.; Balusu, S.; Gorityala, N.; Dandu, L.; Kaur, R. Functional genomic analysis of Candida glabrata-macrophage interaction: Role of chromatin remodeling in virulence. PLoS Pathog. 2012, 8, e1002863. [Google Scholar] [CrossRef] [Green Version]
- Kasper, L.; Seider, K.; Gerwien, F.; Allert, S.; Brunke, S.; Schwarzmuller, T.; Ames, L.; Zubiria-Barrera, C.; Mansour, M.K.; Becken, U.; et al. Identification of Candida glabrata genes involved in pH modulation and modification of the phagosomal environment in macrophages. PLoS ONE 2014, 9, e96015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, M.; Battu, A.; Kaur, R. Aspartyl proteases in Candida glabrata are required for suppression of the host innate immune response. J. Biol. Chem. 2018, 293, 6410–6433. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, I.D.; Brunke, S.; Seider, K.; Schwarzmuller, T.; Firon, A.; d’Enfert, C.; Kuchler, K.; Hube, B. Candida glabrata persistence in mice does not depend on host immunosuppression and is unaffected by fungal amino acid auxotrophy. Infect. Immun. 2010, 78, 1066–1077. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.M.; Shen, H.; Zhang, T.; Huang, X.; Liu, X.Q.; Guo, S.Y.; Zhao, J.J.; Wang, C.F.; Yan, L.; Xu, G.T.; et al. Dectin-1 plays an important role in host defense against systemic Candida glabrata infection. Virulence 2017, 8, 1643–1656. [Google Scholar] [CrossRef] [Green Version]
- Nash, E.E.; Peters, B.M.; Lilly, E.A.; Noverr, M.C.; Fidel, P.L., Jr. A murine model of Candida glabrata vaginitis shows no evidence of an inflammatory immunopathogenic response. PLoS ONE 2016, 11, e0147969. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Lowes, D.J.; Barker, K.S.; Palmer, G.E.; Peters, B.M. Comparative analysis of the capacity of the Candida species to elicit vaginal immunopathology. Infect. Immun. 2018, 86, e00527-18. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jackson, W.C.; Steinbach, P.A.; Tsien, R.Y. Evolution of new nonantibody proteins via iterative somatic hypermutation. Proc. Natl. Acad. Sci. USA 2004, 101, 16745–16749. [Google Scholar] [CrossRef] [Green Version]
- Gurskaya, N.G.; Fradkov, A.F.; Terskikh, A.; Matz, M.V.; Labas, Y.A.; Martynov, V.I.; Yanushevich, Y.G.; Lukyanov, K.A.; Lukyanov, S.A. GFP-like chromoproteins as a source of far-red fluorescent proteins. FEBS Lett. 2001, 507, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, A.C.; Seman, B.G.; Hammond, J.H.; Archambault, L.S.; Hogan, D.A.; Wheeler, R.T. Candida albicans and Pseudomonas aeruginosa interact to enhance virulence of mucosal infection in transparent zebrafish. Infect. Immun. 2017, 85, e00475-17. [Google Scholar] [CrossRef] [Green Version]
- Chapuis, A.F.; Ballou, E.R.; MacCallum, D.M. A bright future for fluorescence imaging of fungi in living hosts. J. Fungi 2019, 5, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, M.; Battu, A.; Kaur, R. Host-pathogen interaction in Candida glabrata infection: Current knowledge and implications for antifungal therapy. Expert Rev. Anti Infect. Ther. 2020, 18, 1093–1103. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schrevens, S.; Sanglard, D. Investigating Candida glabrata Urinary Tract Infections (UTIs) in Mice Using Bioluminescence Imaging. J. Fungi 2021, 7, 844. https://doi.org/10.3390/jof7100844
Schrevens S, Sanglard D. Investigating Candida glabrata Urinary Tract Infections (UTIs) in Mice Using Bioluminescence Imaging. Journal of Fungi. 2021; 7(10):844. https://doi.org/10.3390/jof7100844
Chicago/Turabian StyleSchrevens, Sanne, and Dominique Sanglard. 2021. "Investigating Candida glabrata Urinary Tract Infections (UTIs) in Mice Using Bioluminescence Imaging" Journal of Fungi 7, no. 10: 844. https://doi.org/10.3390/jof7100844