Biotransformation of Animal Fat-By Products into ARA-Enriched Fermented Bioproducts by Solid-State Fermentation of Mortierella alpina
Abstract
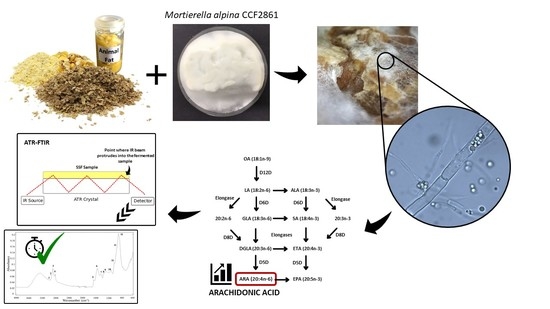
:1. Introduction
2. Materials and Methods
2.1. Production Microorganism and Preparation of Spore Suspension
2.2. Conditions of Solid-State Fermentation
2.3. Preparation of AF Emulsion
2.4. Humidity and Substrate Utilization Analysis
2.5. Estimation of Fungal Biomass in Fermented Bioproduct
2.6. Microspopic Observation of Fungal Mycelia during SSF
2.7. Analysis of Fatty Acid Profile Andf Content in Fermented Bioproduct
2.8. Lipid Isolation and Analysis of Lipid Classes by TLC
2.9. ATR-FTIR Spectroscopy Analysis
2.10. Data Analysis
3. Results
3.1. Fungal Growth and Aubstrate Utilization during SSF
3.2. Microscopic Observation of SSF Process
3.3. The Impact of AF Supplementation on the Humidity and pH of the Fermented Bioproducts
3.4. Lipid Profile and Accumulation
3.5. Arachidonic Acid Yield
3.6. Biochemical Profile of the Fermented Bioproducts Obtained by FTIR-ATR Spectroscopy
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woodgate, L.S.; van der Veen, J.T. Fats and Oils—Animal Based. In Food Processing: Principles and Applications, 2nd ed.; Clark, S., Jung, S., Lamsal, B., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2004; pp. 481–499. [Google Scholar]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012. Available online: http://dx.doi.org/10.6027/Nord2014-002 (accessed on 20 August 2020).
- Doppenberg, J.; van der Aar, P.J.; van Vuure, C. Animal fat: Nutritious ingredient for animal diets. All Feed 2015, 23, 9–11. [Google Scholar]
- Antimanon, S.; Chamkhuy, W.; Sutthiwattanakul, S.; Laoteng, K. Efficient production of arachidonic acid of Mortierella sp. by solid-state fermentation using combinatorial medium with spent mushroom substrate. Chem. Pap. 2018, 72, 2899–2908. [Google Scholar] [CrossRef]
- Szotkowski, M.; Byrtusova, D.; Haronikova, A.; Vysoka, M.; Rapta, M.; Shapaval, V.; Marova, I. Study of metabolic adaptation of red yeasts to waste animal fat substrate. Microorganisms 2019, 7, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel production from oleaginous microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P.S. Recent developments and innovations in solid state fermentation. Biotech. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Hölker, U.; Höfer, M.; Lenz, J. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Z.; Duan, Y.; Chen, H. Relations between substrate morphological change and oxygen transfer in solid-state fermentation (SSF) using Penicillium decumbens JUA10. J. Chem. Technol. Biotechnol. 2014, 89, 1582–1589. [Google Scholar] [CrossRef]
- Čertík, M.; Klempová, T.; Guothová, L.; Mihálik, D.; Kraic, J. Biotechnology for the functional improvement of cereal-based materials enriched with PUFA and pigments. Eur. J. Lipid Sci. Technol. 2013, 115, 1247–1256. [Google Scholar] [CrossRef]
- Postemsky, P.D.; Curvetto, N.R. Solid-state fermentation of cereal grains and sunflower seed hulls by Grifola gargal and Grifola sordulenta. Int. Biodeter. Biodegr. 2015, 100, 52–61. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E.-H. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with Zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef]
- Kaur, P.; Ghoshal, G.; Jain, A. Bio-utilization of fruits and vegetables waste to produce β-carotene in solid-state fermentation: Characterization and antioxidant activity. Process Biochem. 2019, 76, 155–164. [Google Scholar] [CrossRef]
- Chandra, M.S.; Viswanath, B.; Reddy, R.B. Cellulolytic enzymes on lignocellulosic substrates in solid state fermentation by Aspergillus niger. Indian J. Microbiol. 2007, 47, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klempová, T.; Slaný, O.; Šišmiš, M.; Marcinčák, S.; Čertík, M. Dual production of polyunsaturated fatty acids and beta-carotene with Mucor wosnessenskii by the process of solid-state fermentation using agro-industrial waste. J. Biotechnol. 2020, 311, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Slaný, O.; Klempová, T.; Marcinčák, S.; Čertík, M. Production of high-value bioproducts enriched with γ-linolenic acid and β-carotene by filamentous fungi Umbelopsis isabellina using solid-state fermentations. Ann. Microbiol. 2020, 70, 5. [Google Scholar] [CrossRef]
- Asadi, S.Z.; Khosravi-Darani, K.; Nikoopour, H.; Bakhoda, H. Evaluation of the effect of process variables on the fatty acid profile of single cell oil produced by Mortierella using solid-state fermentation. Crit. Rev. Biotechnol. 2015, 35, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Tao, J.; Luo, Y.; Tang, T.; Miao, J.; Yang, Q. Microbial oil production from solid-state fermentation by a newly isolated oleaginous fungus, Mucor circinelloides Q531 from mulberry branches. R. Soc. Open Sci. 2018, 5, 180551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, G.; Guan, Z.; Liu, F.G.; Liao, X.; Cai, Y. Arachidonic acid production by Mortierella alpina using raw crop materials. Acta Sci. Pol. Technol. Aliment. 2015, 14, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Kikukawa, H.; Sakuradani, E.; Ando, A.; Shimizu, S.; Ogawa, J. Arachidonic acid production by the oleaginous fungus Mortierella alpina 1S-4: A review. J. Adv. Res. 2018, 11, 15–22. [Google Scholar] [CrossRef]
- Mironov, A.A.; Nemashkalov, V.A.; Stepanova, N.N.; Kamzolova, S.V.; Rymowicz, W.; Morgunov, I.G. The Effect of pH and Temperature on Arachidonic Acid Production by Glycerol-Grown Mortierella alpina NRRL-A-10995. Fermentation 2018, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Čertík, M.; Sakuradani, E.; Shimizu, S. Desaturase-defective fungal mutants: Useful tools for the regulation and overproduction of polyunsaturated fatty acids. Trends Biotechnol. 1998, 16, 500–505. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, O. Development and validation of a GC–FID method for quantitative analysis of oleic acid and related fatty acids. J. Pharm. Anal. 2015, 5, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamani, A.; Jeihanipour, A.; Edebo, L.; Niklasson, C.; Taherzadeh, M.J. Determination of glucosamine and N-acetyl glucosamine in fungal cell walls. J. Agric. Food Chem. 2008, 56, 8314–8318. [Google Scholar] [CrossRef] [PubMed]
- Forfang, K.; Zimmermann, B.; Kosa, G.; Kohler, A.; Shapaval, V. FTIR Spectroscopy for Evaluation and Monitoring of Lipid Extraction Efficiency for Oleaginous Fungi. PLoS ONE 2017, 12, e0170611. [Google Scholar] [CrossRef] [Green Version]
- Kosa, G.; Zimmermann, B.; Kohler, A.; Ekeberg, D.; Afseth, N.K.; Mounier, J.; Shapaval, V. High-throughput screening of Mucoromycota fungi for production of low- and high value lipids. Biotechnol. Biofuels 2018, 11. [Google Scholar] [CrossRef]
- Shapaval, V.; Afseth, N.K.; Vogt, G.; Kohler, A. Fourier transform infrared spectroscopy for the prediction of fatty acid profiles in Mucor fungi grown in media with different carbon sources. Microb. Cell Fact. 2014, 13, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapaval, V.; Brandenburg, J.; Blomqvist, J.; Tafintseva, V.; Passoth, V.; Sandgren, M.; Kohler, A. Biochemical profiling, prediction of total lipid content and fatty acid profile in oleaginous yeasts by FTIR spectroscopy. Biotechnol. Biofuels 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Tamilvanan, S. Oil-in-water lipid emulsions: Implications for parenteral and ocular delivering systems. Prog. Lipid Res. 2004, 43, 489–533. [Google Scholar] [CrossRef]
- Aidoo, K.; Hendry, R.; Wood, B.J.B. Estimation of fungal growth in solid state fermentation system. Appl. Microbiol. Biot. 1981, 12, 6–9. [Google Scholar] [CrossRef]
- Katano, H.; Takakuwa, M.; Hayakawa, H.; Kimoto, H. Determination of Chitin Based on the Colorimetric Assay of Glucosamine in Acidic Hydrolysate. Anal. Sci. 2016, 32, 701–703. [Google Scholar] [CrossRef] [Green Version]
- Čertík, M.; Shimizu, S. Kinetic analysis of oil biosynthesis by an arachidonic acid-producing fungus, Mortierella alpina 1S-4. Appl. Microbiol. Biotechnol. 2000, 54, 224–230. [Google Scholar] [CrossRef]
- Gajdoš, P.; Nicaud, J.-M.; Rossignol, T.; Čertík, M. Single cell oil production on molasses by Yarrowia lipolytica strains overexpressing DGA2 in multicopy. Appl. Microbiol. Biotechnol. 2015, 99, 8065–8074. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Kohler, A.; Kirschner, C.; Oust, A.; Martens, H. Extended multiplicative signal correction as a tool for separation and characterization of physical and chemical information in Fourier transform infrared microscopy images of cryo-sections of beef loin. Appl. Spectrosc. 2005, 59, 707–716. [Google Scholar] [CrossRef]
- Zimmermann, B.; Kohler, A. Optimizing Savitzky-Golay parameters for improving spectral resolution and quantification in infrared spectroscopy. Appl. Spectrosc. 2013, 67, 892–902. [Google Scholar] [CrossRef] [Green Version]
- Demšar, U.; Harris, P.; Brunsdon, C.; Fotheringham, A.; Mcloone, S. Principal Component Analysis on Spatial Data: An Overview. Ann. Am. Assoc. Geogr. 2013, 103, 106–128. [Google Scholar] [CrossRef]
- Massarolo, K.C.; Ferreira, C.F.J.; de Borba, V.S.; Kupski, L.; Furlong, E.B. Particle size and physical-chemical characteristics of hydrothermally treated cornmeal on resistant starch content. Food Chem. 2019, 283, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Merali, Z.; Collins, S.R.A.; Elliston, A.; Wilson, D.R.; Käsper, A.; Waldron, K.W. Characterization of cell wall components of wheat bran following hydrothermal pretreatment and fractionation. Biotechnol. Biofuels 2015, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyal, S.D.; Narine, S.S. Implications for the use of Mortierella fungi in the industrial production of essential fatty acids. Food Res. Int. 2005, 38, 445–467. [Google Scholar] [CrossRef]



| FA | [%] |
|---|---|
| C14:0 | 2.23 |
| C16:0 | 25.66 |
| C16:1 n-7 | 2.18 |
| C18:0 | 21.33 |
| C18:1 n-9 | 38.34 |
| C18:1 n-7 | 1.94 |
| C18:2 n-6 | 5.47 |
| C18:3 n-3 | 0.81 |
| C20:0 | 0.23 |
| Other fatty acids | 1.81 |
| Lipid Structure | |
| Polar lipids | 0.14 |
| Monoacylglycerols | 0.28 |
| Diacylglycerols | 2.08 |
| Sterol structures | 9.38 |
| Free fatty acids | 7.20 |
| Triacylglycerols | 66.54 |
| Esterified sterols | 11.23 |
| Other lipid structures | 3.15 |
| Cereal Matrix | Tween®® 40 [%(w/w)] | Animal Fat [%(w/w)] |
|---|---|---|
| cornmeal | 0 | 0 1 |
| 0.5 | 0 2 | |
| 1 | 0 2 | |
| 2 | 0 2 | |
| 3 | 0 2 | |
| 0.5 | 5 3 | |
| 1 | 10 3 | |
| 2 | 20 3 | |
| 3 | 30 3 | |
| wheat bran | 0 | 0 1 |
| 0.5 | 0 2 | |
| 1 | 0 2 | |
| 2 | 0 2 | |
| 3 | 0 2 | |
| 0.5 | 5 3 | |
| 1 | 10 3 | |
| 2 | 20 3 | |
| 3 | 30 3 |
| Substrate Utilization [%] | Substrate Humidity [%] | pH | FBM [mg/g BP] | |
|---|---|---|---|---|
| cornmeal (CM) | 22.3 ± 0.9 | 60.0 ± 0.5 | 5.4 ± 0.1 | 241.3 ± 10.5 |
| CM + 0.5% Tween 40 | 23.1 ± 0.5 | 58.1 ± 0.6 | 5.5 ± 0.0 | 244.0 ± 26.8 |
| CM + 1% Tween 40 | 25.8 ± 0.5 | 60.1 ± 0.7 | 5.3 ± 0.1 | 272.8 ± 13.5 |
| CM + 2% Tween 40 | 24.3 ± 0.6 | 59.0 ± 1.5 | 5.3 ± 0.3 | 286.2 ± 6.1 |
| CM + 3% Tween 40 | 23.7 ± 1.0 | 58.6 ± 0.8 | 5.2 ± 0.1 | 289.3 ± 13.8 |
| CM + 0.5% Tween 40 + 5% AF | 21.1 ± 1.4 | 53.2 ± 0.6 | 5.2 ± 0.0 | 137.1 ± 15.6 |
| CM + 1% Tween 40 + 10% AF | 17.6 ± 0.4 | 48.8 ± 1.3 | 4.6 ± 0.1 | 120.7 ± 10.3 |
| CM + 2% Tween 40 + 20% AF | 13.1 ± 0.4 | 40.0 ± 1.1 | 4.5 ± 0.2 | 82.7 ± 14.8 |
| CM + 3% Tween 40 + 30% AF | 10.7 ± 0.4 | 36.5 ± 1.5 | 4.3 ± 0.1 | 73.7 ± 6.0 |
| wheat bran (WB) | 13.2 ± 0.5 | 58.7 ± 1.9 | 5.5 ± 0.1 | 188.8 ± 1.4 |
| WB + 0.5% Tween 40 | 12.3 ± 0.6 | 58.9 ± 0.1 | 5.1 ± 0.5 | 174.2 ± 10.4 |
| WB + 1% Tween 40 | 12.5 ± 0.4 | 56.8 ± 1.9 | 5.3 ± 0.2 | 193.6 ± 5.2 |
| WB + 2% Tween 40 | 12.6 ± 0.7 | 58.4 ± 0.2 | 5.2 ± 0.2 | 168.1 ± 5.9 |
| WB + 3% Tween 40 | 13.6 ± 0.6 | 54.2 ± 4.5 | 5.4 ± 0.3 | 166.0 ± 1.1 |
| WB + 0.5% Tween 40 + 5% AF | 10.4 ± 0.4 | 54.4 ± 1.0 | 5.1 ± 0.2 | 205.0 ± 1.1 |
| WB + 1% Tween 40 + 10% AF | 9.6 ± 0.2 | 48.7 ± 1.6 | 4.8 ± 0.1 | 189.9 ± 4.8 |
| WB + 2% Tween 40 + 20% AF | 9.3 ± 0.7 | 44.2 ± 2.2 | 4.5 ± 0.1 | 122.7 ± 5.1 |
| WB + 3% Tween 40 + 30% AF | 8.8 ± 0.5 | 39.1 ± 0.7 | 4.3 ± 0.0 | 92.2 ± 5.2 |
| TFA | Fatty Acids (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%/BP) | C14:0 | C16:0 | C16:1, n-7 | C18:0 | C18:1, n-9 | C18:1, n-7 | C18:2, n-6 | C18:3, n-6 | C18:3, n-3 | C20:3, n-6 | C20:4, n-6 | Others | |
| cornmeal | |||||||||||||
| CM nf | 3.2 | 0.6 | 12.7 | nd | 2.5 | 27.3 | 0.7 | 54.7 | nd | 1.5 | nd | nd | nd |
| CM f | 13.8 | 0.8 | 14.5 | 0.1 | 5.6 | 13.7 | 0.7 | 23.8 | 2.3 | 0.5 | 2.5 | 26.4 | 9.0 |
| +0.5%Tween 40 nf | 3.7 | 0.7 | 14.3 | nd | 2.4 | 26.2 | 0.7 | 54.1 | nd | 1.5 | nd | nd | nd |
| +0.5%Tween 40 f | 13.7 | 0.6 | 15.6 | 0.1 | 5.8 | 13.2 | 1.0 | 23.5 | 2.4 | 0.3 | 2.5 | 27.5 | 7.6 |
| +1%Tween 40 nf | 3.4 | 0.8 | 16.6 | nd | 2.6 | 25.4 | 0.7 | 52.4 | nd | 1.6 | nd | nd | nd |
| +1%Tween 40 f | 13.4 | 0.8 | 16.0 | 0.1 | 5.7 | 12.8 | 0.6 | 22.6 | 2.3 | 0.4 | 2.6 | 27.1 | 8.9 |
| +2%Tween 40 nf | 4.3 | 1.0 | 20.3 | nd | 2.6 | 23.7 | 0.6 | 50.2 | nd | 1.5 | nd | nd | nd |
| +2%Tween 40 f | 13.8 | 0.8 | 17.3 | 0.1 | 5.8 | 13.0 | 0.6 | 22.7 | 2.3 | 0.5 | 2.5 | 26.1 | 8.4 |
| +3%Tween 40 nf | 4.7 | 0.6 | 23.9 | nd | 2.8 | 22.3 | 0.6 | 48.3 | nd | 1.3 | nd | nd | nd |
| +3%Tween 40 f | 13.7 | 0.8 | 18.2 | 0.1 | 5.7 | 12.7 | 0.6 | 23.0 | 2.3 | 0.5 | 2.5 | 25.4 | 8.2 |
| +0.5%T + 5%AF nf | 7.1 | 0.7 | 18.0 | 0.8 | 7.8 | 30.9 | 1.1 | 39.4 | nd | 1.3 | nd | nd | nd |
| +0.5%T + 5%AF f | 14.2 | 1.0 | 17.1 | 0.6 | 8.8 | 18.0 | 1.2 | 18.6 | 2.1 | 0.6 | 1.8 | 22.6 | 7.5 |
| +1%T + 10%AF nf | 11.3 | 1.2 | 22.2 | 1.7 | 12.0 | 33.4 | 1.5 | 26.9 | nd | 1.2 | nd | nd | nd |
| +1%T + 10%AF f | 16.1 | 1.1 | 18.8 | 1.0 | 11.3 | 21.1 | 1.5 | 16.4 | 2.0 | 0.6 | 1.4 | 18.3 | 6.4 |
| +2%T + 20%AF nf | 18.1 | 1.7 | 24.7 | 2.0 | 15.0 | 36.4 | 1.7 | 17.5 | nd | 1.1 | nd | nd | nd |
| +2%T + 20%AF f | 19.0 | 1.4 | 21.0 | 1.3 | 13.5 | 24.8 | 1.7 | 12.9 | 1.6 | 0.7 | 1.1 | 14.6 | 5.3 |
| +3%T + 30%AF nf | 24.3 | 1.9 | 25.8 | 2.1 | 16.6 | 37.4 | 1.8 | 13.3 | nd | 1.0 | nd | nd | nd |
| +3%T + 30%AF f | 24.7 | 1.6 | 22.4 | 1.6 | 14.7 | 30.4 | 1.7 | 12.7 | 1.2 | 0.8 | 0.7 | 8.6 | 3.6 |
| wheat bran | |||||||||||||
| WB nf | 3.5 | 1.7 | 17.1 | nd | 1.0 | 17.3 | 1.5 | 56.1 | nd | 4.5 | nd | nd | 0.7 |
| WB f | 3.1 | 0.2 | 12.6 | 0.1 | 1.6 | 15.3 | 1.1 | 38.6 | 2.2 | 2.3 | 0.6 | 20.4 | 5.2 |
| +0.5%Tween 40 nf | 3.6 | nd | 20.7 | nd | 1.1 | 16.6 | 1.5 | 54.7 | nd | 4.4 | nd | nd | 0.9 |
| +0.5%Tween 40 f | 3.4 | 0.1 | 13.9 | nd | 1.5 | 15.9 | 1.1 | 38.2 | 1.8 | 2.6 | 0.3 | 20.5 | 4.1 |
| +1%Tween 40 nf | 3.8 | nd | 21.8 | 0.2 | 1.2 | 16.7 | 1.4 | 53.4 | nd | 4.2 | nd | nd | 1.2 |
| +1%Tween 40 f | 3.3 | 0.3 | 15.7 | nd | 1.7 | 14.4 | 1.1 | 36.8 | 2.3 | 2.2 | 0.6 | 20.6 | 4.3 |
| +2%Tween 40 nf | 3.8 | nd | 26.7 | nd | 1.5 | 15.8 | 1.4 | 50.1 | nd | 3.9 | nd | nd | 0.7 |
| +2%Tween 40 f | 3.6 | 0.3 | 15.5 | nd | 2.1 | 13.8 | 1.1 | 34.9 | 2.4 | 2.1 | 0.2 | 22.3 | 5.5 |
| +3%Tween 40 nf | 4.6 | 0.2 | 33.6 | 0.2 | 1.8 | 13.7 | 1.2 | 44.9 | nd | 3.5 | nd | nd | 1.0 |
| +3%Tween 40 f | 4.0 | 0.3 | 18.8 | 0.2 | 2.3 | 13.0 | 1.0 | 31.5 | 2.1 | 2.0 | 0.7 | 23.1 | 5.1 |
| +0.5%T + 5%AF nf | 6.6 | 1.0 | 22.1 | 1.0 | 9.5 | 26.5 | 1.6 | 34.6 | nd | 2.9 | nd | nd | 0.8 |
| +0.5%T + 5%AF f | 5.6 | 0.5 | 17.2 | 0.7 | 7.1 | 18.2 | 1.9 | 21.8 | 3.5 | 1.2 | 0.8 | 19.6 | 7.4 |
| +1%T + 10%AF nf | 9.9 | 1.2 | 23.7 | 1.2 | 10.6 | 27.2 | 1.7 | 30.7 | nd | 2.6 | nd | nd | 1.1 |
| +1%T + 10%AF f | 7.6 | 0.8 | 17.7 | 1.0 | 8.6 | 22.9 | 1.91 | 17.7 | 3.6 | 1.2 | 0.8 | 18.1 | 5.9 |
| +2%T + 20%AF nf | 14.4 | 1.8 | 25.8 | 1.7 | 12.8 | 31.1 | 1.8 | 22.2 | nd | 2.0 | Nd | nd | 1.1 |
| +2%T + 20%AF f | 11.4 | 1.1 | 19.6 | 1.3 | 11.6 | 24.2 | 2.0 | 13.8 | 4.0 | 1.0 | 0.9 | 15.6 | 5.9 |
| +3%T + 30%AF nf | 23.4 | 1.3 | 26.1 | 1.6 | 16.0 | 33.3 | 1.8 | 17.1 | nd | 1.6 | nd | nd | 0.9 |
| +3%T + 30%AF f | 17.0 | 1.0 | 19.8 | 1.3 | 13.3 | 25.9 | 2.0 | 13.6 | 3.7 | 1.0 | 0.8 | 12.6 | 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slaný, O.; Klempová, T.; Shapaval, V.; Zimmermann, B.; Kohler, A.; Čertík, M. Biotransformation of Animal Fat-By Products into ARA-Enriched Fermented Bioproducts by Solid-State Fermentation of Mortierella alpina. J. Fungi 2020, 6, 236. https://doi.org/10.3390/jof6040236
Slaný O, Klempová T, Shapaval V, Zimmermann B, Kohler A, Čertík M. Biotransformation of Animal Fat-By Products into ARA-Enriched Fermented Bioproducts by Solid-State Fermentation of Mortierella alpina. Journal of Fungi. 2020; 6(4):236. https://doi.org/10.3390/jof6040236
Chicago/Turabian StyleSlaný, Ondrej, Tatiana Klempová, Volha Shapaval, Boris Zimmermann, Achim Kohler, and Milan Čertík. 2020. "Biotransformation of Animal Fat-By Products into ARA-Enriched Fermented Bioproducts by Solid-State Fermentation of Mortierella alpina" Journal of Fungi 6, no. 4: 236. https://doi.org/10.3390/jof6040236