Comparative Analysis of Heart Regeneration: Searching for the Key to Heal the Heart—Part I: Experimental Injury Models to Study Cardiac Regeneration
Abstract
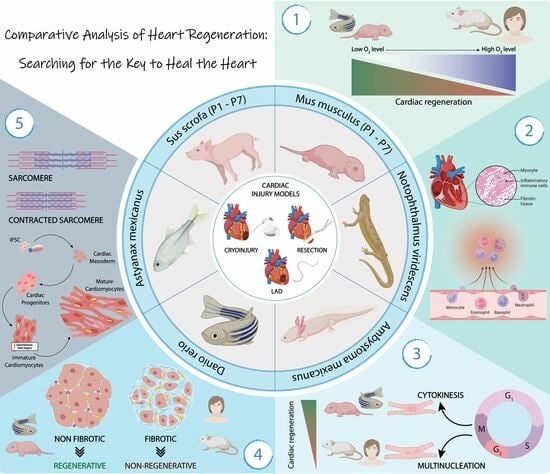
:1. Introduction
2. Experimental Models of Cardiac Regeneration
3. Injury Models to Study Cardiac Healing
4. Heart Regeneration after Injury in Different Animal Models
4.1. Invertebrate Models
4.2. Vertebrate Models
- -
- Fish models
- -
- Amphibian models
- -
- Chicken models
- -
- Mammal models
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus. 23 July 2020. Available online: https://www.cureus.com/articles/36728-global-epidemiology-of-ischemic-heart-disease-results-from-the-global-burden-of-disease-study (accessed on 20 July 2023).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. In International Review of Cell and Molecular Biology, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 298, 229p. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Barrabes, J.A.; Bøtker, H.E.; Davidson, S.M.; Di Lisa, F.; Downey, J.; Engstrom, T.; Ferdinandy, P.; Carbrera-Fuentes, H.A.; Heusch, G.; et al. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Res. Cardiol. 2016, 111, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/reperfusion injury following acute myocardial infarction: A critical issue for clinicians and forensic pathologists. Mediat. Inflamm. 2017, 2017, 7018393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, R.B.; Sommers, H.M.; Smyth, G.A.; Flack, H.A.; Linn, H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch. Pathol. 1960, 70, 68–78. [Google Scholar]
- Kathiresan, S.; Srivastava, D. Genetics of human cardiovascular disease. Cell 2012, 148, 1242–1257. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Yu, H.; Zwartbol, M.; Ruifrok, W.P.; van Gilst, W.H.; de Boer, R.A.; Silljé, H.H.W. Identification of hypertrophy- and heart failure-associated genes by combining in vitro and in vivo models. Physiol. Genom. 2012, 44, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, K.; Cai, Z.; Gupta, R.; Parajuli, N.; Fox-Talbot, K.; Darshan, M.S.; Gonzalez, F.J.; Semenza, G.L. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning. Proc. Natl. Acad. Sci. USA 2012, 109, 10504–10509. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Natl. Inst. Health 2009, 324, 98–102. [Google Scholar] [CrossRef] [Green Version]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, M.H. Changes in Regenerative Capacity through Lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivien, C.J.; Hudson, J.E.; Porrello, E.R. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen. Med. 2016, 1, 16012. [Google Scholar] [CrossRef] [Green Version]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.O.; Chapin, S.; Sherry, R. Regeneration of the ventricular myocardium in amphibians. Nature 1974, 248, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Soonpaa, M.H.; Field, L.J. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am. J. Physiol. Circ. Physiol. 1997, 272, H220–H226. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Chong, N.; Ellis, B.; Ren, X.; Senapati, S.; Chang, H.C.; Zorlutuna, P. Constant-potential environment for activating and synchronizing cardiomyocyte colonies with on-chip ion-depleting perm-selective membranes. Lab A Chip 2020, 20, 4273–4284. [Google Scholar] [CrossRef]
- Ellis, B.W.; Dmitry, O.; Traktuev; Merfeld-Clauss, S.; Can, U.I.; Wang, M.; Bergeron, R.; Zorlutuna, P.; March, K.L. Adipose Stem Cell Secretome Markedly Improves Rodent Heart and hiPSC-derived Cardiomyocyte Recovery from Cardioplegic Transport Solution Exposure. Stem Cells 2021, 39, 170–182. [Google Scholar] [CrossRef]
- He, L.; Zhou, B. Cardiomyocyte proliferation: Remove brakes and push accelerators. Cell Res. 2017, 27, 959–960. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.T.; Ye, S.; Su, J.; Garg, V. Cardiomyocyte Proliferation and Maturation: Two Sides of the Same Coin for Heart Regeneration. Front. Cell Dev. Biol. 2020, 8, 594226. [Google Scholar] [CrossRef]
- Peter, A.K.; Bjerke, M.A.; Leinwand, L.A. Biology of the cardiac myocyte in heart disease. Mol. Biol. Cell 2016, 27, 2149–2160. [Google Scholar] [CrossRef]
- Watanabe, M.; Horie, H.; Kurata, Y.; Inoue, Y.; Notsu, T.; Wakimizu, T.; Adachi, M.; Yamamoto, K.; Morikawa, K.; Kuwabara, M.; et al. Esm1 and Stc1 as angiogenic factors responsible for protective actions of adipose-derived stem cell sheets on chronic heart failure after rat myocardial infarction. Circ. J. 2021, 85, 657–666. [Google Scholar] [CrossRef]
- Choi, S.-C.; Seo, H.-R.; Cui, L.-H.; Song, M.-H.; Noh, J.-M.; Kim, K.-S.; Choi, J.-H.; Kim, J.-H.; Park, C.-Y.; Joo, H.J.; et al. Modeling hypoxic stress in vitro using human embryonic stem cells derived cardiomyocytes matured by fgf4 and ascorbic acid treatment. Cells 2021, 10, 2741. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.W.; Acun, A.; Isik Can, U.; Zorlutuna, P. Human IPSC-derived myocardium-on-chip with capillary-like flow for personalized medicine. Biomicrofluidics 2017, 11, 024105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basara, G.; Gulberk Ozcebe, S.; Ellis, B.W.; Zorlutuna, P. Tunable human myocardium derived decellularized extracellular matrix for 3d bioprinting and cardiac tissue engineering. Gels 2021, 7, 70. [Google Scholar] [CrossRef]
- Ren, X.; Ellis, B.W.; Ronan, G.; Blood, S.R.; DeShetler, C.; Senapati, S.; March, K.L.; Handberg, E.; Anderson, D.; Pepine, C.; et al. A multiplexed ion-exchange membrane-based miRNA (MIX·miR) detection platform for rapid diagnosis of myocardial infarction. Lab A Chip 2021, 21, 3876–3887. [Google Scholar] [CrossRef]
- Gentile, C. Filling the gaps between the in vivo and in vitro microenviron- ment: Engineering of spheroids for stem cell technology. Curr. Stem Cell Res. Ther. 2016, 11, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Vunjak-Novakovic, G. In vitro models of ischemia-reperfusion injury. Regen. Eng. Transl. Med. 2018, 4, 142–153. [Google Scholar] [CrossRef]
- Sharma, P.; Wang, X.; Ming, C.L.C.; Vettori, L.; Figtree, G.; Boyle, A.; Gentile, C. Considerations for the Bioengineering of Advanced Cardiac In Vitro Models of Myocardial Infarction. Small 2021, 17, e2003765. [Google Scholar] [CrossRef]
- Williams, M.A.C.; Mair, D.B.; Lee, W.; Lee, E.; Kim, D.H. Engineering Three-Dimensional Vascularized Cardiac Tissues. Tissue Eng. Part B Rev. 2022, 28, 336–350. [Google Scholar] [CrossRef]
- Wanjare, M.; Kawamura, M.; Hu, C.; Alcazar, C.; Wang, H.; Woo, Y.J.; Huang, N.F. Vascularization of Engineered Spatially Patterned Myocardial Tissue Derived from Human Pluripotent Stem Cells in vivo. Front. Bioeng. Biotechnol. 2019, 7, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorba, I.; Mostert, D.; Hermans, L.H.L.; van der Pol, A.; Kurniawan, N.A.; Bouten, C.V.C. In Vitro Methods to Model Cardiac Mechanobiology in Health and Disease.Tissue Engineering. Tissue Eng. Part C Methods 2021, 27, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Bolli, R.; Canty, J.M., Jr.; Du, X.-J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dvornikov, A.V.; Huttner, I.G.; Ma, X.; Santiago, C.F.; Fatkin, D.; Xu, X. A Langendorff-like system to quantify cardiac pump function in adult zebrafish. DMM Dis. Model. Mech. 2018, 11, dmm034819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Liu, C.; Bao, M.; Liu, W.; Nie, Y.; Lian, H.; Hu, S. Optimized Langendorff perfusion system for cardiomyocyte isolation in adult mouse heart. J. Cell. Mol. Med. 2020, 24, 14619–14625. [Google Scholar] [CrossRef]
- Rossello, X.; Hall, A.R.; Bell, R.M.; Yellon, D.M. Characterization of the Langendorff Perfused Isolated Mouse Heart Model of Global Ischemia-Reperfusion Injury: Impact of Ischemia and Reperfusion Length on Infarct Size and LDH Release. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 286–295. [Google Scholar] [CrossRef]
- Alqarni, F.; Alsaadi, M.; Karem, F. MR image analysis of ex-vivo mouse model of heart ischemia. Saudi J. Biol. Sci. 2021, 28, 1990–1998. [Google Scholar] [CrossRef]
- Montero-Bullon, J.F.; Aveiro, S.S.; Melo, T.; Martins-Marques, T.; Lopes, D.; Neves, B.; Girão, H.; Rosário MDomingues, M.; Domingues, P. Cardiac phospholipidome is altered during ischemia and reperfusion in an ex vivo rat model. Biochem. Biophys. Rep. 2021, 27, 101037. [Google Scholar] [CrossRef]
- Dittrich, A.; Lauridsen, H. Myocardial infarction and the immune response—Scarring or regeneration? A comparative look at mammals and popular regenerating animal models. J. Immunol. Regen. Med. 2019, 4, 100016. [Google Scholar] [CrossRef]
- Laube, F.; Heister, M.; Scholz, C.; Borchardt, T.; Braun, T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J. Cell Sci. 2006, 119, 4719–4729. [Google Scholar] [CrossRef] [Green Version]
- González-Rosa, J.M.; Burns, C.E.; Burns, C.G. Zebrafish heart regeneration: 15 years of discoveries. Regeneration 2017, 4, 105–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poss, K.D.; Keating, M.T.; Nechiporuk, A. Tales of regeneration in zebrafish. Dev. Dyn. 2003, 226, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; D’agostino, G.; Loo, S.J.; Wang, C.X.; Su, L.P.; Tan, S.H.; Tee, G.Z.; Pua, C.J.; Pena, E.M.; Cheng, R.B.; et al. Early regenerative capacity in the porcine heart. Circulation 2018, 138, 2798–2808. [Google Scholar] [CrossRef]
- Agnew, E.J.; Velayutham, N.; Ortiz, G.M.; Alfieri, C.M.; Hortells, L.; Moore, V.; Riggs, K.W.; Baker, R.S.; Gibson, A.M.; Ponny, S.R.; et al. Scar formation with decreased cardiac function following ischemia/reperfusion injury in 1 month old swine. J. Cardiovasc. Dev. Dis. 2020, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Haubner, B.J.; Adamowicz-Brice, M.; Khadayate, S.; Tiefenthaler, V.; Metzler, B.; Aitman, T.; Penninger, J.M. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging 2012, 4, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Darehzereshki, A.; Rubin, N.; Gamba, L.; Kim, J.; Fraser, J.; Huang, Y.; Billings, J.; Mohammadzadeh, R.; Wood, J.; Warburton, D.; et al. Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev. Biol. 2015, 399, 91–99. [Google Scholar] [CrossRef]
- Cox, J.L.; Malaisrie, S.C.; Churyla, A.; Metha, C.; Kruse, J.; Kislitsina, O.; McCarthy, P.M. Cryosurgery for Atrial Fibrillation: Physiologic Basis for Creating Optimal Cryolesions. Ann. Thorac. Surg. 2020, 112, 354–362. [Google Scholar] [CrossRef]
- Mahmoud, A.I.; Porrello, E.R.; Kimura, W.; Olson, E.N.; Sadek, H.A. Surgical models for cardiac regeneration in neonatal mice. Nat. Protoc. 2014, 9, 305–311. [Google Scholar] [CrossRef]
- Bei, Y.; Chen, C.; Hua, X.; Yin, M.; Meng, X.; Huang, Z.; Qi, W.; Su, Z.; Liu, C.; Lehmann, H.I.; et al. A modified apical resection model with high accuracy and reproducibility in neonatal mouse and rat hearts. Npj Regen. Med. 2023, 8, 9. [Google Scholar] [CrossRef]
- Kolk, M.V.V.; Meyberg, D.; Deuse, T.; Tang-Quan, K.R.; Robbins, R.C.; Reichenspurner, H.; Schrepfer, S. LAD-Ligation: A Murine Model of Myocardial Infarction. J. Vis. Exp. 2009, 32, 1438. [Google Scholar]
- Gamba, L.; Harrison, M.; Lien, C.L. Cardiac regeneration in model organisms. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 288. [Google Scholar] [CrossRef] [Green Version]
- Rubin, N.; Harrison, M.; Krainock, M.; Kim, R.; Lien, C.L. Recent advancements in understanding endogenous heart regeneration-insights from adult zebrafish and neonatal mice. Semin. Cell Dev. Biol. 2016, 58, 34–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbern, J.C.; Mummery, C.L.; Lee, R.T. Model Systems for Cardiovascular Regenerative Biology. Cold Spring Harb. Perspect. Med. 2013, 3, a014019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, P.C.; Gungi, A.; Unni, M. Cellular and Molecular Mechanisms of Hydra Regeneration. In Evo-Devo: Non-Model Species in Cell and Developmental Biology; Results and Problems in Cell Differentiation; Tworzydlo, W., Bilinski, S.M., Eds.; Springer International Publishing: Cham, Germany, 2019; Volume 68, pp. 259–290. Available online: http://link.springer.com/10.1007/978-3-030-23459-1_12 (accessed on 28 July 2023).
- Jopling, C.; Sleep, E.; Raya, M.; Martí, M.; Raya, A.; Izpisúa Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef] [Green Version]
- Chablais, F.; Veit, J.; Rainer, G.; Jaźwińska, A. The zebrafish heart regenerates after cryoinjury- induced myocardial infarction. BMC Dev. Biol. 2011, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- González-Rosa, J.M.; Martín, V.; Peralta, M.; Torres, M.; Mercader, N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 2011, 138, 1663–1674. [Google Scholar] [CrossRef] [Green Version]
- Hein, S.J.; Lehmann, L.H.; Kossack, M.; Juergensen, L.; Fuchs, D.; Katus, H.A.; Hassel, D. Advanced Echocardiography in Adult Zebrafish Reveals Delayed Recovery of Heart Function after Myocardial Cryoinjury. PLoS ONE 2015, 10, e0122665. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Panáková, D.; Kikuchi, K.; Holdway, J.E.; Gemberling, M.; Burris, J.S.; Singh, S.P.; Dickson, A.L.; Lin, Y.-F.; Sabeh, M.K.; et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 2011, 138, 3421–3430. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef]
- Manuel, G.-R.J.; Michka, S.; Dorothy, F.; Mark, H.S.; Loren, J.F.; Burns, C.E.; Geoffrey, C. Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish Article Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev. Cell 2018, 44, 433–446. [Google Scholar]
- Wetsel, M.R.; Hart, R.W.; Rawleigh, E.W. Mexican eyeless Characin fishes, genus Astyanax: Environment, distribution, and evolution. KIP Monogr. 1977. Available online: https://digitalcommons.usf.edu/kip_monographs/17/ (accessed on 20 July 2023).
- Gross, J.B. The complex origin of Astyanax cavefish. BMC Evol. Biol. 2012, 12, 105. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, W.R. Regressive Evolution in Astyanax Cavefish. Annu. Rev. Genet. 2009, 43, 25–47. [Google Scholar] [CrossRef] [Green Version]
- Stockdale, W.T.; Lemieux, M.E.; Killen, A.C.; Zhao, J.; Hu, Z.; Riepsaame, J.; Hamilton, N.; Kudoh, T.; Riley, P.R.; van Aerle, R.; et al. Heart Regeneration in the Mexican Cavefish. Cell Rep. 2018, 25, 1997–2007.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Morioka, M.; Kimura, S.; Tasaki, M.; Inohaya, K.; Kudo, A. Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Dev. Dyn. 2014, 243, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Holdway, J.E.; Major, R.J.; Blum, N.; Dahn, R.D.; Begemann, G.; Poss, K.D. Retinoic Acid Production by Endocardium and Epicardium Is an Injury Response Essential for Zebrafish Heart Regeneration. Dev. Cell 2011, 20, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Cano-Martínez, A.; Vargas-González, A.; Guarner-Lans, V.; Prado-Zayago, E.; León-Oleda, M.; Nieto-Lima, B. Functional and structural regeneration in the axolotl heart (Ambystoma mexicanum) after partial ventricular amputation. Arch. Cardiol. Mex. 2010, 80, 21147570. [Google Scholar]
- Lauridsen, H.; Pedersen, M. Circulating cells contribute to cardiac regeneration in the axolotl. FASEB J. 2015, 29, 1029.14. [Google Scholar] [CrossRef]
- Jacobs, G.F.M.; Michielsen, R.P.A.; Kühn, E.R. Thyroxine and Triiodothyronine in Plasma and Thyroids of the Neotenic and Metamorphosed Axolotl Ambystoma mexicanurn: Influence of TRH Injections. Gen. Comp. Endocrinol. 1998, 70, 145–151. [Google Scholar] [CrossRef]
- Monaghan, J.R.; Stier, A.C.; Michonneau, F.; Smith, M.D.; Pasch, B.; Maden, M.; Seifert, A.W. Experimentally induced metamorphosis in axolotls reduces regenerative rate and fidelity. Regeneration 2014, 1, 2–14. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. Metamorphosis and the amphibian immune system. Immunol. Rev. 1998, 166, 221–230. [Google Scholar] [CrossRef]
- Godwin, J.W.; Rosenthal, N. Scar-free wound healing and regeneration in amphibians: Immunological influences on regenerative success. Differentiation 2014, 87, 66–75. [Google Scholar] [CrossRef]
- Hirose, K.; Payumo, A.Y.; Cutie, S.; Hoang, A.; Zhang, H.; Guyot, R.; Lunn, D.; Bigley, R.B.; Yu, H.; Wang, J.; et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 2019, 364, 184–188. [Google Scholar] [CrossRef]
- Oberpriller, J.O.; Oberpriller, J.C. Response of the adult newt ventricle to injury. J. Exp. Zool. 1974, 187, 249–253. [Google Scholar] [CrossRef]
- Witman, N.; Murtuza, B.; Davis, B.; Arner, A.; Morrison, J.I. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev. Biol. 2011, 354, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piatkowski, T.; Mühlfeld, C.; Borchardt, T.; Braun, T.; Chong, J.J.; Reinecke, H.; Iwata, M.; Torok-Storb, B.; Stempien-Otero, A.; Murry, C.E.; et al. Reconstitution of the Myocardium in Regenerating Newt Hearts is Preceded by Transient Deposition of Extracellular Matrix Components. Stem Cells Dev. 2013, 22, 1921–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, S.E.; Odelberg, S.J.; Simon, H.-G. A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev. Biol. 2013, 382, 457–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, C.M.A.; Molina, A.G.; Zapata, J.D.; Delgado, J.P. Limb regeneration in a direct-developing terrestrial salamander, Bolitoglossa ramosi (Caudata : Plethodontidae) Limb regeneration in plethodontid salamanders. Regeneration 2017, 4, 227–235. [Google Scholar] [CrossRef]
- Rumyantsev, P.P. Growth and hyperplasia of cardiac muscle cells. Sov. Med. Rev. 1991. Available online: https://www.taylorfrancis.com/books/mono/10.4324/9781315076652/growth-hyperplasia-cardiac-muscle-cells-rumyantsev (accessed on 20 July 2023).
- Novikov, A.I.; Khloponin, P.A. O reparativnykh protsessakh v émbrional’nom i postémbrional’nom miokardiogeneze Gallus domesticus L. [Reparative processes during embryonal and postembryonal myocardiogenesis in Gallus domesticus L. Arkh Anat. Gistol. Embriol. 1982, 82, 59–67. [Google Scholar] [PubMed]
- Gunadasa-Rohling, M.; Masters, M.; Maguire, M.L.; Smart, S.C.; Schneider, J.E.; Riley, P.R. Magnetic Resonance Imaging of the Regenerating Neonatal Mouse Heart. Circulation 2018, 138, 2439–2441. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Strungs, E.G.; Ongstad, E.L.; O’Quinn, M.P.; Palatinus, J.A.; Jourdan, L.J.; Gourdie, R.G. Cryoinjury models of the adult and neonatal mouse heart for studies of scarring and regeneration. Methods Mol. Biol. 2013, 1037, 343–353. [Google Scholar]
- Sophy, A.J.; Michele, A.S.; Frank, K.L.; Martin, B.; Michael, H.; Shaun, R.; Jane, C.L.; Robert, M.D.; Alexander, Y.; Bernd, F.; et al. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. USA 2012, 109, 13380–13385. [Google Scholar]
- Bryant, D.M.; O’Meara, C.C.; Ho, N.N.; Gannon, J.; Cai, L.; Lee, R.T. A systematic analysis of neonatal mouse heart regeneration after apical resection. J. Mol. Cell. Cardiol. 2015, 79, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Mario, N.; Antoni, V.-R.; Sylvia, J.B.-G.; Ignasi, J.; Lola, M.; Daniel, N.; Mercè, M.; Ángel, R. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Cell Biol. 2018, 4, eaao5553 2. [Google Scholar]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.; Liccardo, D.; LaCanna, R.; Zhang, X.; Lu, R.; Finck, B.N.; Leigh, T.; Chen, X.; Drosatos, K.; Tian, Y. Fatty Acid Oxidation Promotes Cardiomyocyte Proliferation Rate but Does Not Change Cardiomyocyte Number in Infant Mice. Front. Cell Dev. Biol. 2019, 7, 42. [Google Scholar] [CrossRef]
- Patterson, M.; Barske, L.; Van Handel, B.; Rau, C.D.; Gan, P.; Sharma, A.; Parikh, S.; Denholtz, M.; Huang, Y.; Yamaguchi, Y.; et al. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat. Genet. 2017, 49, 1346–1353. [Google Scholar] [CrossRef]
- Murugan, S.J.; Gnanapragasam, J.; Vettukattil, J. Acute myocardial infarction in the neonatal period. Cardiol. Young 2002, 12, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Cesna, S.; Eicken, A.; Juenger, H.; Hess, J. Successful treatment of a newborn with acute myocardial infarction on the first day of life. Pediatr. Cardiol. 2013, 34, 1868–1870. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, A.P.; Urbanek, K.; Kajstura, J.; Yan, S.M.; Finato, N.; Bussani, R.; Nadal-Ginard, B.; Silvestri, F.; Leri, A.; Beltrami, C.A.; et al. Evidence that human cardiac myocytes divide after myocardial infarction. N. Engl. J. Med. 2001, 344, 1750–1757. [Google Scholar] [CrossRef]
- Saker, D.M.; Walsh-Sukys, M.; Spector, M.; Zahka, K.G. Cardiac Recovery and Survival After Neonatal Myocardial Infarction. Pediatr. Cardiol. 1997, 18, 139–142. [Google Scholar] [CrossRef]
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.-I.; Penninger, J.M. New Hypotheses in Clinical Medicine Functional: Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ. Res. 2016, 118, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Fratz, S.; Hager, A.; Schreiber, C.; Schwaiger, M.; Hess, J.; Stern, H.C. Long-Term Myocardial Scarring After Operation for Anomalous Left Coronary Artery From the Pulmonary Artery. Ann. Thorac. Surg. 2011, 92, 1761–1765. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Casas, J.M.; Caño-Carrillo, S.; Sánchez-Fernández, C.; Franco, D.; Lozano-Velasco, E. Comparative Analysis of Heart Regeneration: Searching for the Key to Heal the Heart—Part I: Experimental Injury Models to Study Cardiac Regeneration. J. Cardiovasc. Dev. Dis. 2023, 10, 325. https://doi.org/10.3390/jcdd10080325
Castillo-Casas JM, Caño-Carrillo S, Sánchez-Fernández C, Franco D, Lozano-Velasco E. Comparative Analysis of Heart Regeneration: Searching for the Key to Heal the Heart—Part I: Experimental Injury Models to Study Cardiac Regeneration. Journal of Cardiovascular Development and Disease. 2023; 10(8):325. https://doi.org/10.3390/jcdd10080325
Chicago/Turabian StyleCastillo-Casas, Juan Manuel, Sheila Caño-Carrillo, Cristina Sánchez-Fernández, Diego Franco, and Estefanía Lozano-Velasco. 2023. "Comparative Analysis of Heart Regeneration: Searching for the Key to Heal the Heart—Part I: Experimental Injury Models to Study Cardiac Regeneration" Journal of Cardiovascular Development and Disease 10, no. 8: 325. https://doi.org/10.3390/jcdd10080325