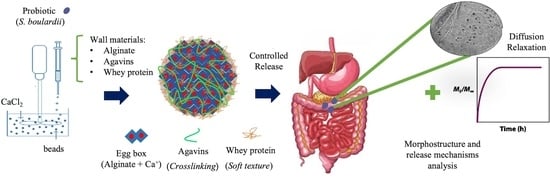
Kinetics and Mechanisms of Saccharomyces boulardii Release from Optimized Whey Protein–Agavin–Alginate Beads under Simulated Gastrointestinal Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Standard Inoculum for Encapsulation
2.3. Solution Preparation of Agavins and Whey Protein
2.4. Encapsulation of Yeast by Ionic Gelation
2.5. Viability of Encapsulated Saccharomyces boulardii
2.6. Optimization of the Morphostructure of Beads
Morphostructural Parameters by Microscopy
2.7. Physicochemical Properties of the Capsules
2.8. Survival of Saccharomyces boulardii under In Vitro Gastrointestinal Digestion Conditions
2.8.1. Viability
2.8.2. Kinetics and Release Mechanisms
2.9. Statistical Analysis
3. Results
3.1. Growth of Saccharomyces boulardii
3.2. Viability of Encapsulated Saccharomyces boulardii
3.3. Morphostructural Characterization of the Beads
3.3.1. Morphostructural Optimization of the Beads
3.3.2. Study of the Internal Morphostructure (Mesostructure) of the Beads
3.3.3. Study of the External Morphostructure of the Beads
3.4. Physicochemical Properties of Capsules
3.4.1. Conformational Analysis by Fourier Transform Infrared Spectroscopy (FT-IR)
3.4.2. Thermal Properties by Differential Scanning Calorimetry (DSC)
3.5. Survival of Saccharomyces boulardii under In Vitro Gastrointestinal Digestion Conditions
3.5.1. Viability of Saccharomyces boulardii
3.5.2. Kinetics and Release Mechanisms of Saccharomyces boulardii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fakhrullin, R.F.; Lvov, Y.M. “Face-Lifting” and “Make-up” for Microorganisms: Layer-by-Layer Polyelectrolyte Nanocoating. ACS Nano 2012, 6, 4557–4564. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Khan, A.; Khan, R.A.; Riedl, B.; Lacroix, M. Encapsulation of Probiotic Bacteria in Biopolymeric System. Crit. Rev. Food Sci. Nutr. 2013, 53, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Beaussart, A.; Alsteens, D.; Dupres, V.; Claes, I.; Von Ossowski, I.; De Vos, W.M.; Palva, A.; Lebeer, S.; Vanderleyden, J.; et al. Adhesion and Nanomechanics of Pili from the Probiotic Lactobacillus Rhamnosus GG. ACS Nano 2013, 7, 3685–3697. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-Layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in Microencapsulation of Probiotics: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A Review of the Microencapsulation Techniques for the Incorporation of Probiotic Bacteria in Functional Foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1515–1536. [Google Scholar] [CrossRef]
- Leong, J.Y.; Lam, W.H.; Ho, K.W.; Voo, W.P.; Lee, M.F.X.; Lim, H.P.; Lim, S.L.; Tey, B.T.; Poncelet, D.; Chan, E.S. Advances in Fabricating Spherical Alginate Hydrogels with Controlled Particle Designs by Ionotropic Gelation as Encapsulation Systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Naranjo-Durán, A.M.; Quintero-Quiroz, J.; Rojas-Camargo, J.; Ciro-Gómez, G.L. Modified-Release of Encapsulated Bioactive Compounds from Annatto Seeds Produced by Optimized Ionic Gelation Techniques. Sci. Rep. 2021, 11, 1317. [Google Scholar] [CrossRef]
- Alkhatib, H.; Doolaanea, A.A.; Assadpour, E.; Mohmad Sabere, A.S.; Mohamed, F.; Jafari, S.M. Optimizing the Encapsulation of Black Seed Oil into Alginate Beads by Ionic Gelation. J. Food Eng. 2022, 328, 111065. [Google Scholar] [CrossRef]
- Goma-Tchimbakala, E.J.C.D.; Pietrini, I.; Dal Bello, F.; Goma-Tchimbakala, J.; Lo Russo, S.; Corgnati, S.P. Great Abilities of Shinella Zoogloeoides Strain from a Landfarming Soil for Crude Oil Degradation and a Synergy Model for Alginate-Bead-Entrapped Consortium Efficiency. Microorganisms 2022, 10, 1361. [Google Scholar] [CrossRef]
- Arslan, S.; Erbas, M.; Tontul, I.; Topuz, A. Microencapsulation of Probiotic Saccharomyces Cerevisiae Var: Boulardii with Different Wall Materials by Spray Drying. LWT—Food Sci. Technol. 2015, 63, 685–690. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Omura, M.H.; Cedran, M.F.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; Garcia, S. Effect of Natural Polymers on the Survival of Lactobacillus Casei Encapsulated in Alginate Microspheres. J. Microencapsul. 2017, 34, 431–439. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds; Springer: Berlin/Heidelberg, Germany, 2014; Volume 7, ISBN 1239301491067. [Google Scholar]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Camelo-Méndez, G.A.; Vanegas-Espinoza, P.E.; Jiménez-Aparicio, A.R.; Bello-Pérez, L.A.; Del Villar-Martínez, A.A. Morphometric Characterization of Chalkiness in Mexican Rice Varieties by Digital Image Analysis and Multivariate Discrimination. Rev. Mex. Ing. Quím. 2013, 12, 371–378. [Google Scholar]
- Hristu, R.; Eftimie, L.G.; Stanciu, S.G.; Tranca, D.E.; Paun, B.; Sajin, M.; Stanciu, G.A. Quantitative Second Harmonic Generation Microscopy for the Structural Characterization of Capsular Collagen in Thyroid Neoplasms. Biomed. Opt. Express 2018, 9, 3923–3936. [Google Scholar] [CrossRef] [PubMed]
- Chanona-Pérez, J.J.; Quevedo, R.; Jimenez-Aparicio, A.R.; Gumeta-Chávez, C.; Mendoza-Pérez, G.; Calderón-Domínguez, G.; Alamilla-Beltrán, L.; Gutierrez-López, G.F. Image Processing Methods and Fractal Analysis for Quantitative Evaluation of Size, Shape, Structure and Microstructure in Food Materials. In Food Engineering Integrated Approaches; Springer: Berlin/Heidelberg, Germany, 2006; Volume 1999, pp. 1–6. ISBN 9780387754291. [Google Scholar]
- Quintanilla-Carvajal, M.X.; Meraz-Torres, L.S.; Alamilla-Beltrán, L.; Chanona-Pérez, J.J.; Terres-Rojas, E.; Hernández-Sánchez, H.; Gutiérrez-López, G.F. Morphometric Characterization of Spray—Dried Microcapsules before and after α—Tocopherol Extraction. Rev. Mex. Ing. Quím. 2011, 10, 301–312. [Google Scholar]
- Flores, F.P.; Kong, F. In Vitro Release Kinetics of Microencapsulated Materials and the Effect of the Food Matrix. Annu. Rev. Food Sci. Technol. 2017, 8, 237–259. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of Diffusion Controlled Drug Delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Argin, S.; Kofinas, P.; Lo, Y.M. The Cell Release Kinetics and the Swelling Behavior of Physically Crosslinked Xanthan-Chitosan Hydrogels in Simulated Gastrointestinal Conditions. Food Hydrocoll. 2014, 40, 138–144. [Google Scholar] [CrossRef]
- Dima, C.; Pətraşcu, L.; Cantaragiu, A.; Alexe, P.; Dima, Ş. The Kinetics of the Swelling Process and the Release Mechanisms of Coriandrum sativum L. Essential Oil from Chitosan/Alginate/Inulin Microcapsules. Food Chem. 2016, 195, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Malekjani, N.; Jafari, S.M. Modeling the Release of Food Bioactive Ingredients from Carriers/Nanocarriers by the Empirical, Semiempirical, and Mechanistic Models. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3–47. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.C.L.C.; Alexandrino, F.; Marcelino, H.R.; Picciani, P.H.d.S.; e Silva, K.G.d.H.; Genre, J.; de Oliveira, A.G.; do Egito, E.S.T. Understanding Drug Release Data through Thermodynamic Analysis. Materials 2017, 10, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; McGinty, S.; Pontrelli, G.; Zhou, L. Theoretical Model for Diffusion-Reaction Based Drug Delivery from a Multilayer Spherical Capsule. Int. J. Heat Mass Transf. 2022, 183, 122072. [Google Scholar] [CrossRef]
- Hébrard, G.; Hoffart, V.; Beyssac, E.; Cardot, J.M.; Alric, M.; Subirade, M. Coated Whey Protein/Alginate Microparticles as Oral Controlled Delivery Systems for Probiotic Yeast. J. Microencapsul. 2010, 27, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Duongthingoc, D.; George, P.; Katopo, L.; Gorczyca, E.; Kasapis, S. Effect of Whey Protein Agglomeration on Spray Dried Microcapsules Containing Saccharomyces boulardii. Food Chem. 2013, 141, 1782–1788. [Google Scholar] [CrossRef]
- Avila-Reyes, S.V.; Garcia-Suarez, F.J.; Jiménez, M.T.; San Martín-Gonzalez, M.F.; Bello-Perez, L.A. Protection of L. Rhamnosus by Spray-Drying Using Two Prebiotics Colloids to Enhance the Viability. Carbohydr. Polym. 2014, 102, 423–430. [Google Scholar] [CrossRef]
- Cancino-Castillo, L.A.; Beristain, C.I.; Pascual-Pineda, L.A.; Ortiz-Basurto, R.I.; Juárez-Trujillo, N.; Jiménez-Fernández, M. Effective Microencapsulation of Enterococcus Faecium in Biopolymeric Matrices Using Spray Drying. Appl. Microbiol. Biotechnol. 2020, 104, 9595–9605. [Google Scholar] [CrossRef]
- Alvarado-Jasso, G.M.; Camacho-Díaz, B.H.; Arenas Ocampo, M.L.; Jiménez-Ferrer, J.E.; Mora-Escobedo, R.; Osorio-Díaz, P. Prebiotic Effects of a Mixture of Agavins and Green Banana Flour in a Mouse Model of Obesity. J. Funct. Foods 2020, 64, 103685. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Urías-Silvas, J.E.; Morales-Hernández, N. The Role of Agave Fructans in Health and Food Applications: A Review. Trends Food Sci. Technol. 2021, 114, 585–598. [Google Scholar] [CrossRef]
- Wack, M.; Blaschek, W. Determination of the Structure and Degree of Polymerisation of Fructans from Echinacea Purpurea Roots. Carbohydr. Res. 2006, 341, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Domínguez, L.; Alamilla-Beltrán, L.; Calderón-Domínguez, G.; Jiménez-Aparicio, A.; Gutiérrez-López, G.; Azuara-Nieto, E. Determinación del punto de solubilización total e incipiente de fructanos extraídos de A. tequilana Weber var. azul. Rev. Mex. Ing. Quim. 2018, 17, 379–388. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Garcia-Hernandez, M.H.; Delgado-Portales, R.E.; Corral-Fernandez, N.E.; Cortez-Espinosa, N.; Ruiz-Cabrera, M.A.; Portales-Perez, D.P. In Vitro Assessment of Agave Fructans (Agave Salmiana) as Prebiotics and Immune System Activators. Int. J. Biol. Macromol. 2014, 63, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Andrade, A.I.; Rivera-Bautista, C.; Godínez-Hernández, C.I.; Ruiz-Cabrera, M.A.; Fuentes-Ahumada, C.; García Chávez, E.; Grajales Lagunes, A. Physiometabolic Effects of Agave Salmiana Fructans Evaluated in Wistar Rats. Int. J. Biol. Macromol. 2018, 108, 1300–1309. [Google Scholar] [CrossRef]
- Crispín-Isidro, G.; Lobato-Calleros, C.; Espinosa-Andrews, H.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of Inulin and Agave Fructans Addition on the Rheological, Microstructural and Sensory Properties of Reduced-Fat Stirred Yogurt. LWT—Food Sci. Technol. 2015, 62, 438–444. [Google Scholar] [CrossRef]
- Campelo-Felix, P.H.; Souza, H.J.B.; Figueiredo, J.d.A.; Fernandes, R.V.d.B.; Botrel, D.A.; de Oliveira, C.R.; Yoshida, M.I.; Borges, S.V. Prebiotic Carbohydrates: Effect on Reconstitution, Storage, Release, and Antioxidant Properties of Lime Essential Oil Microparticles. J. Agric. Food Chem. 2017, 65, 445–453. [Google Scholar] [CrossRef]
- Bermúdez-Quiñones, G.; Ochoa-Martínez, L.A.; Gallegos-Infante, J.A.; Rutiaga-Quiñones, O.M.; Lara-Ceniceros, T.E.; Delgado-Licon, E.; González-Herrera, S.M. Synbiotic Microcapsules Using Agavins and Inulin as Wall Materials for Lactobacillus Casei and Bifidobacterium Breve: Viability, Physicochemical Properties, and Resistance to in Vitro Oro-Gastrointestinal Transit. J. Food Process. Preserv. 2021, 45, e16106. [Google Scholar] [CrossRef]
- Kong, B.; Xiong, Y.L.; Cui, X.; Zhao, X. Hydroxyl Radical-Stressed Whey Protein Isolate: Functional and Rheological Properties. Food Bioprocess Technol. 2013, 6, 169–176. [Google Scholar] [CrossRef]
- Alehosseini, A.; Gomez del Pulgar, E.M.; Fabra, M.J.; Gómez-Mascaraque, L.G.; Benítez-Páez, A.; Sarabi-Jamab, M.; Ghorani, B.; Lopez-Rubio, A. Agarose-Based Freeze-Dried Capsules Prepared by the Oil-Induced Biphasic Hydrogel Particle Formation Approach for the Protection of Sensitive Probiotic Bacteria. Food Hydrocoll. 2019, 87, 487–496. [Google Scholar] [CrossRef]
- De Araújo Etchepare, M.; Nunes, G.L.; Nicoloso, B.R.; Barin, J.S.; Moraes Flores, E.M.; de Oliveira Mello, R.; Ragagnin de Menezes, C. Improvement of the Viability of Encapsulated Probiotics Using Whey Proteins. LWT—Food Sci. Technol. 2020, 117, 108601. [Google Scholar] [CrossRef]
- Buitrago-Arias, C.; Londoño-Moreno, A.; Avila-Reyes, S.V.; Arenas-Ocampo, M.L.; Alamilla-Beltran, L.; Jimenez-Aparicio, A.R.; Camacho-Diaz, B.H. Evaluation of the Fermentation of Acetylated Agave Fructans (Agavins), with Saccharomyces boulardii as a Probiotic. Rev. Mex. Ing. Quím. 2021, 20, 1–13. [Google Scholar] [CrossRef]
- FAO/WHO Probiotics in Food. Health and Nutrition Properties o Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; FAO/WHO Probiotics in Food: Cordoba, Argentina, 2001. [Google Scholar]
- Lapsiri, W.; Bhandari, B.; Wanchaitanawong, P. Viability of Lactobacillus Plantarum TISTR 2075 in Different Protectants during Spray Drying and Storage. Dry. Technol. 2012, 30, 1407–1412. [Google Scholar] [CrossRef]
- Saarela, M.; Mogensen, G.; Fondén, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic Bacteria: Safety, Functional and Technological Properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- Guslandi, M.; Mezzi, G.; Sorghi, M.; Testoni, P.A. Saccharomyces boulardii in Maintenance Treatment of Crohn’s Disease. Dig. Dis. Sci. 2000, 45, 1462–1464. [Google Scholar] [CrossRef]
- Candelli, M.; Nista, C.E.; Nestola, M.; Armuzzi, A.; Silveri, N.G.; Gasbarrini, G.; Gasbarrini, A. Saccharomyces Cerevisiae-Associated Diarrhea in an Immunocompetent Patient with Ulcerative Colitis. J. Clin. Gastroenterol. 2003, 135, 97–109. [Google Scholar] [CrossRef]
- Thomas, M.B.; Vaidyanathan, M.; Radhakrishnan, K.; Raichur, A.M. Enhanced Viability of Probiotic Saccharomyces boulardii Encapsulated by Layer-by-Layer Approach in PH Responsive Chitosan-Dextran Sulfate Polyelectrolytes. J. Food Eng. 2014, 136, 1–8. [Google Scholar] [CrossRef]
- Yun, P.; Devahastin, S.; Chiewchan, N. Microstructures of Encapsulates and Their Relations with Encapsulation Efficiency and Controlled Release of Bioactive Constituents: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1768–1799. [Google Scholar] [CrossRef]
- Duongthingoc, D.; George, P.; Gorczyca, E.; Kasapis, S. Studies on the Viability of Saccharomyces boulardii within Microcapsules in Relation to the Thermomechanical Properties of Whey Protein. Food Hydrocoll. 2014, 42, 232–238. [Google Scholar] [CrossRef]
- Meng, K.L.; Lv, X.C.; Che, H.Y.; Li, Y.; Chen, X.L.; Hu, M.X.; Yan, M. Joint Protection Strategies for Saccharomyces boulardii: Exogenous Encapsulation and Endogenous Biofilm Structure. Appl. Microbiol. Biotechnol. 2021, 105, 8469–8479. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Sada, A.; Orlando, P. Fermentative Ability of Alginate-Prebiotic Encapsulated Lactobacillus Acidophilus and Survival under Simulated Gastrointestinal Conditions. J. Funct. Foods 2009, 1, 319–323. [Google Scholar] [CrossRef]
- Darjani, P.; Hosseini Nezhad, M.; Kadkhodaee, R.; Milani, E. Influence of Prebiotic and Coating Materials on Morphology and Survival of a Probiotic Strain of Lactobacillus Casei Exposed to Simulated Gastrointestinal Conditions. LWT—Food Sci. Technol. 2016, 73, 162–167. [Google Scholar] [CrossRef]
- Piacentini, E.; Poerio, T.; Bazzarelli, F.; Giorno, L. Microencapsulation by Membrane Emulsification of Biophenols Recovered from Olive Mill Wastewaters. Membranes 2016, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Banerjee, D.; Chowdhury, R.; Bhattacharya, P. Controlled Release of Microencapsulated Probiotics in Food Matrix. J. Food Eng. 2018, 238, 61–69. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, M.; McClements, D.J.; Liu, X.; Liu, F. Recent Advances in the Design and Fabrication of Probiotic Delivery Systems to Target Intestinal Inflammation. Food Hydrocoll. 2022, 125, 107438. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The Estimation of the Bactericidal Power of the Blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Pedreschi, F.; Mery, D.; Mendoza, F.; Aguilera, J. Classification of Potato Chips Using Pattern Recognition. J. Food Sci. 2004, 69, 405–411. [Google Scholar]
- Postolović, K.S.; Antonijević, M.D.; Ljujić, B.; Miletić Kovačević, M.; Gazdić Janković, M.; Stanić, Z.D. PH-Responsive Hydrogel Beads Based on Alginate, κ-Carrageenan and Poloxamer for Enhanced Curcumin, Natural Bioactive Compound, Encapsulation and Controlled Release Efficiency. Molecules 2022, 27, 4045. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Fernández-González, R.; Muñoz-Barrutia, A.; Ortiz-De-Solorzano, C. 3D Reconstruction of Histological Sections: Application to Mammary Gland Tissue. Microsc. Res. Tech. 2010, 73, 1019–1029. [Google Scholar] [CrossRef]
- Valente, A.J.; Maddalena, L.A.; Robb, E.L.; Moradi, F.; Stuart, J.A. A Simple ImageJ Macro Tool for Analyzing Mitochondrial Network Morphology in Mammalian Cell Culture. Acta Histochem. 2017, 119, 315–326. [Google Scholar] [CrossRef]
- Smoczyński, M. Fractal Analysis of the Microstructure of Milk Powders Produced at Various Temperatures. J. Food Sci. Technol. 2020, 57, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carrión, M.; Hernando, I.; Sotelo-Díaz, I.; Quintanilla-Carvajal, M.X.; Quiles, A. Use of Image Analysis to Evaluate the Effect of High Hydrostatic Pressure and Pasteurization as Preservation Treatments on the Microstructure of Red Sweet Pepper. Innov. Food Sci. Emerg. Technol. 2015, 27, 69–78. [Google Scholar] [CrossRef]
- Taleb, M.F.A. Radiation Synthesis of Polyampholytic and Reversible PH-Responsive Hydrogel and Its Application as Drug Delivery System. Polym. Bull. 2008, 61, 341–351. [Google Scholar] [CrossRef]
- Djebri, N.; Boutahala, M.; Chelali, N.E.; Boukhalfa, N.; Zeroual, L. Enhanced Removal of Cationic Dye by Calcium Alginate/Organobentonite Beads: Modeling, Kinetics, Equilibriums, Thermodynamic and Reusability Studies. Int. J. Biol. Macromol. 2016, 92, 1277–1287. [Google Scholar] [CrossRef]
- Soury, R.; Jabli, M.; Latif, S.; Alenezi, K.M.; El Oudi, M.; Abdulaziz, F.; Teka, S.; El Moll, H.; Haque, A. Synthesis and Characterization of a New Meso-Tetrakis (2,4,6-Trimethylphenyl) Porphyrinto) Zinc(II) Supported Sodium Alginate Gel Beads for Improved Adsorption of Methylene Blue Dye. Int. J. Biol. Macromol. 2022, 202, 161–176. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; López, M.G. Evaluation of Agave Angustifolia Fructans as Fat Replacer in the Cookies Manufacture. LWT—Food Sci. Technol. 2017, 77, 100–109. [Google Scholar] [CrossRef]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J. Feasibility Study on the Use of Attenuated Total Reflectance MIR Spectroscopy to Measure the Fructan Content in Barley. Anal. Methods 2014, 6, 7710–7715. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; Dzul-Cauich, J.G.; López, M.G.; García-Vieyra, M.I. Fructans (Agavins) from Agave Angustifolia and Agave Potatorum as Fat Replacement in Yogurt: Effects on Physicochemical, Rheological, and Sensory Properties. LWT—Food Sci. Technol. 2021, 140, 110846. [Google Scholar] [CrossRef]
- Krimm’, S.; Bandekart, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Chen, X.; Clarke, M.L.; Wang, J.; Chen, Z. Sum Frequency Generation Vibrational Spectroscopy Studies on Molecular Conformation and Orientation of Biological Molecules at Interfaces. Int. J. Heat Mass. Transfer. 2005, 19, 691–713. [Google Scholar] [CrossRef]
- Durán, E.; Churio, O.; Arias, J.L.; Neira-Carrillo, A.; Valenzuela, C. Preparation and Characterization of Novel Edible Matrices Based on Alginate and Whey for Oral Delivery of Iron. Food Hydrocoll. 2020, 98, 105277. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Urias-Silvas, J.E. Thermal Properties of Agave Fructans (Agave Tequilana Weber Var. Azul). Carbohydr. Polym. 2012, 87, 2671–2676. [Google Scholar] [CrossRef]
- Ignot-Gutiérrez, A.; Ortiz-Basurto, R.I.; García-Barradas, O.; Díaz-Ramos, D.I.; Jiménez-Fernández, M. Physicochemical and Functional Properties of Native and Modified Agave Fructans by Acylation. Carbohydr. Polym. 2020, 245, 116529. [Google Scholar] [CrossRef]
- Bank, H.L. Rapid Assessment of Islet Viability with Acridine Orange and Propidium Iodide. Vitr. Cell. Dev. Biol. 1988, 24, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Paarakh, M.P.; Jose, P.A.N.I.; Setty, C.M.; Christoper, G.P. Release Kinetics—Concepts and Applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of Various Polysaccharides (Alginate, Carrageenan, Gums, Chitosan) and Their Combination with Prebiotic Saccharides (Resistant Starch, Lactosucrose, Lactulose) on the Encapsulation of Probiotic Bacteria Lactobacillus Casei 01 Strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Fabela-Morón, M.F.; Porras-Saavedra, R.; Martínez-Velarde, R.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Alamilla-Beltrán, L. Physical-Chemical Properties and Microstructure of Agave Powders Obtained by Spray Drying. In Food Engineering Series; Barbosa-Cánovas, G.V., Ed.; Springer: New York, NY, USA, 2015; pp. 345–351. ISBN 9781493925773. [Google Scholar]
- Souza, M.; Mesquita, A.; Veríssimo, C.; Grosso, C.; Converti, A.; Maciel, M.I. Microencapsulation by Spray Drying of a Functional Product with Mixed Juice of Acerola and Ciriguela Fruits Containing Three Probiotic Lactobacilli. Dry. Technol. 2022, 40, 1185–1195. [Google Scholar] [CrossRef]
- Sharma, M.; Dash, K.K.; Badwaik, L.S. Physicochemical and Release Behaviour of Phytochemical Compounds Based on Black Jamun Pulp Extracts-Filled Alginate Hydrogel Beads through Vibration Dripping Extrusion. Int. J. Biol. Macromol. 2022, 194, 715–725. [Google Scholar] [CrossRef]







| Nomenclature | A (%) | WP (%) |
|---|---|---|
| B (Control alginate) | 0 | 0 |
| AB (Control Agavins) | 5 | 0 |
| WB (Control WP) | 0 | 5 |
| AWB1 | 2.5 | 2.5 |
| AWB2 | 3.75 | 2.5 |
| AWB3 | 5 | 2.5 |
| AWB4 | 2.5 | 3.75 |
| AWB5 | 3.75 | 3.75 |
| AWB6 | 5 | 3.75 |
| AWB7 | 2.5 | 5 |
| AWB8 | 3.75 | 5 |
| AWB9 | 5 | 5 |
| Korsmeyer–Peppas | Higuchi | Peppas–Sahlin | Release Mechanism of S. boulardii | Damköhler Number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | k | R2 | k | R2 | k1 | k2 | R2 | |||
| B | 0.6389 | 0.0135 | 0.5025 | 1.0277 | 0.4063 | −12.429 | 3.1317 | 0.8977 | Diffusion and relaxation (mainly by relaxation) | 0.786 |
| AB | 0.1285 | 0.1650 | 0.4472 | 1.4407 | 0.6039 | −0.6832 | 0.2798 | 0.8538 | 0.837 | |
| WB | 0.3504 | 0.3354 | 0.6190 | 0.8256 | 0.6926 | −10.914 | 2.8105 | 0.9848 | 0.848 | |
| AWB5 | 0.5358 | 0.0254 | 0.6852 | 0.8111 | 0.7371 | −4.5464 | 1.3314 | 0.8877 | 1.063 | |
| AWB6 | 0.4060 | 0.0757 | 0.9098 | 1.3004 | 0.9144 | 1.0697 | −0.2190 | 0.9694 | Diffusion and relaxation (mainly by diffusion) | 1.121 |
| AWB8 | 0.1987 | 0.1644 | 0.9294 | 1.7422 | 0.9014 | 0.3699 | −0.0448 | 0.9315 | 1.135 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Falcón, M.S.; Buitrago-Arias, C.; Avila-Reyes, S.V.; Solorza-Feria, J.; Arenas-Ocampo, M.L.; Camacho-Díaz, B.H.; Jiménez-Aparicio, A.R. Kinetics and Mechanisms of Saccharomyces boulardii Release from Optimized Whey Protein–Agavin–Alginate Beads under Simulated Gastrointestinal Conditions. Bioengineering 2022, 9, 460. https://doi.org/10.3390/bioengineering9090460
Chávez-Falcón MS, Buitrago-Arias C, Avila-Reyes SV, Solorza-Feria J, Arenas-Ocampo ML, Camacho-Díaz BH, Jiménez-Aparicio AR. Kinetics and Mechanisms of Saccharomyces boulardii Release from Optimized Whey Protein–Agavin–Alginate Beads under Simulated Gastrointestinal Conditions. Bioengineering. 2022; 9(9):460. https://doi.org/10.3390/bioengineering9090460
Chicago/Turabian StyleChávez-Falcón, María Sady, Carolina Buitrago-Arias, Sandra Victoria Avila-Reyes, Javier Solorza-Feria, Martha Lucía Arenas-Ocampo, Brenda Hildeliza Camacho-Díaz, and Antonio Ruperto Jiménez-Aparicio. 2022. "Kinetics and Mechanisms of Saccharomyces boulardii Release from Optimized Whey Protein–Agavin–Alginate Beads under Simulated Gastrointestinal Conditions" Bioengineering 9, no. 9: 460. https://doi.org/10.3390/bioengineering9090460