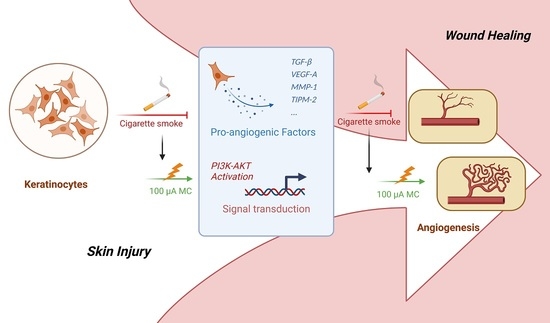
Microcurrent Reverses Cigarette Smoke-Induced Angiogenesis Impairment in Human Keratinocytes In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Cell Lines
2.2. Preparation of Cigarette Smoke Extract (CSE)
2.3. Resazurin Conversion Assay
2.4. Sulforhodamine B (SRB) Staining Assay
2.5. Cell Viability Was Assessed by a Double-Staining Assay Using Calcium-AM/Hoechst 33342
2.6. MC Application
2.7. HaCaT Cells Stimulation and Tube Formation Assay
2.7.1. HaCaT Cells Stimulation and Supernatant Collection
2.7.2. Tube Formation Assay
2.8. Angiogenesis Array Assay
2.9. Identification of Candidate Molecules for Signaling Pathways Using the ChEA3 Tool and the KEGG Database
2.10. Western Blot
2.11. Statistics
3. Results
3.1. 3% CSE Did Not Significanty Affect HaCaT Cells Viability
3.2. 3% CSE Significantly Blunted the Angiogenic Potential by HaCaT Cells, and This Was Offset by 100 μA MC Stimulation
3.3. 100 μA MC Prevents the Detrimental Effects of 3% CSE on the Secretion of Pro-Angiogenic Factors by HaCaT Cells
3.4. Phosphatidyl-Inositol 3-Kinase/Serine-Threonine Kinase (PI3K-Akt), Mitogen-Activated Protein Kinases (MAPK), and Nuclear Factor Kappa-B (NFκB) Signaling Pathway May Be Involved in the Differential Expression of Angiogenic-Related Factors
3.5. MC Exposure Intensified PI3K-Akt and MAPK Signaling in CSE-Injured HaCaT Cells
3.6. Inhibition of PI3K-Akt Signaling Deprived MC of Its Therapeutic Effect on the Tube Formation Function of CSE-Injured HaCaT Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | cigarette smoking |
| CSE | cigarette smoke extract |
| ECL | enhanced chemiluminescence |
| ES | electrical stimulation |
| FBS | fetal bovine serum |
| HaCaT | human keratinocyte cell line |
| MAPK | mitogen-activated protein kinases |
| MC | microcurrent |
| MMP-1 | matrix metalloproteinase-1 |
| NFκB | nuclear factor kappa-B |
| PI3K-Akt | phosphatidyl-inositol 3-kinase/serine-threonine kinase |
| SRB | sulforhodamine B |
| TEP | transepithelial potential difference |
| TGF-β | transforming growth factor beta |
| TIMP-2 | tissue inhibitor of metalloproteinases 2 |
References
- Alberg, A.J. Cigarette smoking: Health effects and control strategies. Drugs Today 2008, 44, 895. [Google Scholar] [CrossRef] [PubMed]
- Effertz, T. Die Kosten des Rauchens in Deutschland im Jahr 2018–aktuelle Situation und langfristige Perspektive. Atemwegs Lungenkrankh. 2019, 45, 307. [Google Scholar] [CrossRef]
- Ockene, I.S.; Miller, N.H. Cigarette smoking, cardiovascular disease, and stroke: A statement for healthcare professionals from the American Heart Association. Circulation 1997, 96, 3243–3247. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.; Dewes, O.; Taufa, N.; Wrapson, W.; Siegert, R. Factors associated with preoperative attrition in bariatric surgery: A protocol for a systematic review. Syst. Rev. 2018, 7, 212. [Google Scholar] [CrossRef]
- Omachi, T.; Sakai, T.; Hiraiwa, H.; Hamada, T.; Ono, Y.; Nakashima, M.; Ishizuka, S.; Matsukawa, T.; Oda, T.; Takamatsu, A. Expression of tenocyte lineage-related factors in regenerated tissue at sites of tendon defect. J. Orthop. Sci. 2015, 20, 380–389. [Google Scholar] [CrossRef]
- Clark, R.A. Basics of cutaneous wound repair. J. Dermatol. Surg. Oncol. 1993, 19, 693–706. [Google Scholar] [CrossRef]
- Honnegowda, T.M.; Kumar, P.; Udupa, E.G.P.; Kumar, S.; Kumar, U.; Rao, P. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast. Aesthetic Res. 2015, 2, 243–249. [Google Scholar]
- Ejaz, S.; Lim, C.W. Toxicological overview of cigarette smoking on angiogenesis. Environ. Toxicol. Pharmacol. 2005, 20, 335–344. [Google Scholar] [CrossRef]
- Chang, C.-J.; Jou, I.-M.; Wu, T.-T.; Su, F.-C.; Tai, T.-W. Cigarette smoke inhalation impairs angiogenesis in early bone healing processes and delays fracture union. Bone Jt. Res. 2020, 9, 99–107. [Google Scholar] [CrossRef]
- Rinderknecht, H.; Nussler, A.K.; Steinestel, K.; Histing, T.; Ehnert, S. Smoking impairs hematoma formation and dysregulates angiogenesis as the first steps of fracture healing. Bioengineering 2022, 9, 186. [Google Scholar] [CrossRef]
- Ud-Din, S.; Bayat, A. Electrical stimulation and cutaneous wound healing: A review of clinical evidence. Wound Care Vol. 2015, 85, 445–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCaig, C. Electrical control of cell behaviour and wound healing. GMS Krankenh. Interdiszip. 2008, 3, Doc03. [Google Scholar]
- Harrison, B.C.; Cazzaniga, A.L.; Davis, S.C.; Mertz, P.M. A wound-isolated Pseudomonas aeruginosa grows a biofilm in vitro within 10 hours and is visualized by light microscopy. Dermatol. Surg. 2003, 29, 631–635. [Google Scholar]
- Zhao, M. Electrical fields in wound healing-an overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Lu, C.; Kolbenschlag, J.; Nüssler, A.K.; Ehnert, S.; McCaig, C.D.; Čebron, U.; Daigeler, A.; Prahm, C. Direct current electrical fields improve experimental wound healing by activation of cytokine secretion and Erk1/2 pathway stimulation. Life 2021, 11, 1195. [Google Scholar] [CrossRef]
- Luo, R.; Dai, J.; Zhang, J.; Li, Z. Accelerated skin wound healing by electrical stimulation. Adv. Healthc. Mater. 2021, 10, 2100557. [Google Scholar] [CrossRef]
- Dictionary, T.F. Microcurrent. Available online: https://medical-dictionary.thefreedictionary.com/microcurrent (accessed on 1 August 2022).
- Nair, H.K. Microcurrent as an adjunct therapy to accelerate chronic wound healing and reduce patient pain. J. Wound Care 2018, 27, 296–306. [Google Scholar] [CrossRef]
- McMakin, C.R.; Oschman, J.L. Visceral and somatic disorders: Tissue softening with frequency-specific microcurrent. J. Altern. Complement. Med. 2013, 19, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Poltawski, L.; Watson, T. Bioelectricity and microcurrent therapy for tissue healing–a narrative review. Phys. Ther. Rev. 2009, 14, 104–114. [Google Scholar] [CrossRef]
- Bravo, M.P.; Soares, G.P.; Daniele de Oliveira, P.; Szezerbaty, S.K.; Frederico, R.C.P.; Maia, L.P. Microcurrent stimulates cell proliferation and modulates cytokine release in fibroblast cells. J. Wound Care 2021, 30, IIIi–IIIix. [Google Scholar] [CrossRef]
- Yu, C.; Hu, Z.-Q.; Peng, R.-Y. Effects and mechanisms of a microcurrent dressing on skin wound healing: A review. Mil. Med. Res. 2014, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Leffmann, D.J.; Arnall, D.A.; Holmgren, P.R.; Cornwall, M.W. Effect of microamperage stimulation on the rate of wound healing in rats: A histological study. Phys. Ther. 1994, 74, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Byl, N.N.; McKenzie, A.L.; West, J.M.; Whitney, J.D.; Hunt, T.K.; Hopf, H.W.; Scheuenstuhl, H. Pulsed microamperage stimulation: A controlled study of healing of surgically induced wounds in Yucatan pigs. Phys. Ther. 1994, 74, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Naeini, A.T.; Oryan, A.; Dehghani, S.; Nikahval, B. Experimental cutaneous wound healing in rabbits: Using continuous microamperage low-voltage electrical stimulation. Comp. Clin. Pathol. 2008, 17, 203–210. [Google Scholar] [CrossRef]
- Kaur, S.; Lyte, P.; Garay, M.; Liebel, F.; Sun, Y.; Liu, J.-C.; Southall, M.D. Galvanic zinc–copper microparticles produce electrical stimulation that reduces the inflammatory and immune responses in skin. Arch. Dermatol. Res. 2011, 303, 551–562. [Google Scholar] [CrossRef]
- Park, R.J.; Son, H.; Kim, K.; Kim, S.; Oh, T. The effect of microcurrent electrical stimulation on the foot blood circulation and pain of diabetic neuropathy. J. Phys. Ther. Sci. 2011, 23, 515–518. [Google Scholar] [CrossRef]
- Blount, A.L.; Foster, S.; Rapp, D.A.; Wilcox, R. The use of bioelectric dressings in skin graft harvest sites: A prospective case series. J. Burn Care Res. 2012, 33, 354–357. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation: A novel tool for tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef]
- Aspera-Werz, R.H.; Ehnert, S.; Heid, D.; Zhu, S.; Chen, T.; Braun, B.; Sreekumar, V.; Arnscheidt, C.; Nussler, A.K. Nicotine and cotinine inhibit catalase and glutathione reductase activity contributing to the impaired osteogenesis of SCP-1 cells exposed to cigarette smoke. Oxidative Med. Cell. Longev. 2018, 2018, 3172480. [Google Scholar] [CrossRef]
- Su, Y.; Han, W.; Giraldo, C.; De Li, Y.; Block, E.R. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 819–825. [Google Scholar] [CrossRef]
- Ehnert, S.; Zhao, J.; Pscherer, S.; Freude, T.; Dooley, S.; Kolk, A.; Stöckle, U.; Nussler, A.K.; Hube, R. Transforming growth factor β1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of Ski-related novel protein N (SnoN): Possible mechanism for the failure of BMP therapy? BMC Med. 2012, 10, 101. [Google Scholar] [CrossRef]
- Ehnert, S.; Baur, J.; Schmitt, A.; Neumaier, M.; Lucke, M.; Dooley, S.; Vester, H.; Wildemann, B.; Stöckle, U.; Nussler, A.K. TGF-β1 as possible link between loss of bone mineral density and chronic inflammation. PLoS ONE 2010, 5, e14073. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Mariappan, N.; Williams, J.G.; Prager, M.D.; Eberhart, R.C. “Engineering” the wound-healing process. IEEE Eng. Med. Biol. Mag. 1999, 18, 22–26. [Google Scholar] [CrossRef]
- Arnaoutova, I.; Kleinman, H.K. In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010, 5, 628–635. [Google Scholar] [CrossRef]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Ehnert, S.; Aspera-Werz, R.H.; Ihle, C.; Trost, M.; Zirn, B.; Flesch, I.; Schröter, S.; Relja, B.; Nussler, A.K. Smoking dependent alterations in bone formation and inflammation represent major risk factors for complications following total joint arthroplasty. J. Clin. Med. 2019, 8, 406. [Google Scholar] [CrossRef]
- Keenan, A.B.; Torre, D.; Lachmann, A.; Leong, A.K.; Wojciechowicz, M.L.; Utti, V.; Jagodnik, K.M.; Kropiwnicki, E.; Wang, Z.; Ma’ayan, A. ChEA3: Transcription factor enrichment analysis by orthogonal omics integration. Nucleic Acids Res. 2019, 47, W212–W224. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Ehnert, S.; Falldorf, K.; Fentz, A.-K.; Ziegler, P.; Schröter, S.; Freude, T.; Ochs, B.G.; Stacke, C.; Ronniger, M.; Sachtleben, J. Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure—Clinical implication possible. Bone Rep. 2015, 3, 48–56. [Google Scholar] [CrossRef]
- Xiao, Q.; Murphy, R.A.; Houston, D.K.; Harris, T.B.; Chow, W.-H.; Park, Y. Dietary and supplemental calcium intakes in relation to mortality from cardiovascular diseases in the NIH-AARP Diet and Health Study. JAMA Intern. Med. 2013, 173, 639. [Google Scholar] [CrossRef] [PubMed]
- Bosselmann, T.; Kolbenschlag, J.; Goertz, O.; Zahn, P.; Prantl, L.; Lehnhardt, M.; Behr, B.; Sogorski, A. Improvement of superficial and deep cutaneous microcirculation due to axillary plexus anesthesia impaired by smoking. J. Clin. Med. 2021, 10, 2114. [Google Scholar] [CrossRef] [PubMed]
- Goertz, O.; Kapalschinski, N.; Skorzinski, T.; Kolbenschlag, J.; Daigeler, A.; Hirsch, T.; Homann, H.; Muehlberger, T. Wound healing complications in smokers, non-smokers and after abstinence from smoking. Der Chir. Z. Fur. Alle Geb. Der Oper. Medizen 2012, 83, 652–656. [Google Scholar]
- Pellaton, C.; Kubli, S.; Feihl, F.; Waeber, B. Blunted vasodilatory responses in the cutaneous microcirculation of cigarette smokers. Am. Heart J. 2002, 144, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Aspera-Werz, R.H.; Chen, T.; Ehnert, S.; Zhu, S.; Fröhlich, T.; Nussler, A.K. Cigarette smoke induces the risk of metabolic bone diseases: Transforming growth factor beta signaling impairment via dysfunctional primary cilia affects migration, proliferation, and differentiation of human mesenchymal stem cells. Int. J. Mol. Sci. 2019, 20, 2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.D.; Buncke, G.; Slezak, S.; Buncke, H.J. Cigarette smoking, plastic surgery, and microsurgery. J. Reconstr. Microsurg. 1996, 12, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Reus, W., 3rd; Colen, L.B.; Straker, D.J. Tobacco smoking and complications in elective microsurgery. Plast. Reconstr. Surg. 1992, 89, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.M.; Maroudas, E.; Osborn, J.L. Sine-wave electrical stimulation initiates a voltage-gated potassium channel-dependent soft tissue response characterized by induction of hemocyte recruitment and collagen deposition. Physiol. Rep. 2016, 4, e12832. [Google Scholar] [CrossRef]
- Edwick, D.O.; Hince, D.A.; Rawlins, J.M.; Wood, F.M.; Edgar, D.W. Does electrical stimulation improve healing in acute minor burn injury, as measured by bioimpedance spectroscopy? A single center, randomized, controlled trial. Burn. Open 2022, 6, 42–50. [Google Scholar] [CrossRef]
- Thawer, H.A.; Houghton, P.E. Effects of electrical stimulation on the histological properties of wounds in diabetic mice. Wound Repair Regen. 2001, 9, 107–115. [Google Scholar] [CrossRef]
- Michaud, S.-É.; Ménard, C.; Guy, L.-G.; Gennaro, G.; Rivard, A. Inhibition of hypoxia-induced angiogenesis by cigarette smoke exposure: Impairment of the HIF-lalpha/VEGF pathway. FASEB J. 2003, 17, 1150–1152. [Google Scholar] [CrossRef]
- Souza, A.K.; Souza, T.R.; das Neves, L.M.S.; Leite, G.d.P.M.F.; Garcia, S.B.; de Jesus Guirro, R.R.; Barbosa, R.I.; de Oliveira Guirro, E.C. Effect of high voltage pulsed current on the integration of total skin grafts in rats submitted to nicotine action. J. Tissue Viability 2019, 28, 161–166. [Google Scholar] [CrossRef]
- Bai, H.; Zhao, M.; Forrester, J.; McCaig, C. Electric stimulation has a direct effect on vascular endothelial cells: Guiding cell elongation, orientation and migration. J. Cell Sci. 2003, 117, 397–405. [Google Scholar]
- Bai, H.; McCaig, C.D.; Forrester, J.V.; Zhao, M. DC electric fields induce distinct preangiogenic responses in microvascular and macrovascular cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1234–1239. [Google Scholar] [CrossRef]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef]
- Ma, L.; Wang, W.; Chow, J.; Yuen, S.; Cho, C. Reduction of EGF is associated with the delay of ulcer healing by cigarette smoking. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G10–G17. [Google Scholar] [CrossRef] [Green Version]
- Roberts, A.B.; Sporn, M.B.; Assoian, R.K.; Smith, J.M.; Roche, N.S.; Wakefield, L.M.; Heine, U.I.; Liotta, L.A.; Falanga, V.; Kehrl, J.H. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 4167–4171. [Google Scholar] [CrossRef]
- Nall, A.V.; Brownlee, R.E.; Colvin, C.P.; Schultz, G.; Fein, D.; Cassisi, N.J.; Nguyen, T.; Kalra, A. Transforming growth factor β1 improves wound healing and random flap survival in normal and irradiated rats. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 171–177. [Google Scholar] [CrossRef]
- Chen, Y.; Aspera-Werz, R.H.; Menger, M.M.; Falldorf, K.; Ronniger, M.; Stacke, C.; Histing, T.; Nussler, A.K.; Ehnert, S. Exposure to 16 Hz pulsed electromagnetic fields protect the structural integrity of primary cilia and associated TGF-β signaling in osteoprogenitor cells harmed by cigarette Smoke. Int. J. Mol. Sci. 2021, 22, 7036. [Google Scholar] [CrossRef]
- Olsson, A.-K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling: In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Maes, C. Role and regulation of vascularization processes in endochondral bones. Calcif. Tissue Int. 2013, 92, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Corral, C.J.; Siddiqui, A.; Wu, L.; Farrell, C.L.; Lyons, D.; Mustoe, T.A. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch. Surg. 1999, 134, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Howdieshell, T.R.; Callaway, D.; Webb, W.L.; Gaines, M.D.; Procter Jr, C.D.; Pollock, J.S.; Brock, T.L.; McNeil, P.L. Antibody neutralization of vascular endothelial growth factor inhibits wound granulation tissue formation. J. Surg. Res. 2001, 96, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C.; Thompson, Z.; Miclau, T.; Werb, Z.; Helms, J.A. Altered fracture repair in the absence of MMP9. Development 2003, 130, 4123. [Google Scholar] [CrossRef]
- Chakraborty, N.; Gautam, A.; Muhie, S.; Miller, S.-A.; Jett, M.; Hammamieh, R. An integrated omics analysis: Impact of microgravity on host response to lipopolysaccharide in vitro. BMC Genom. 2014, 15, 1–14. [Google Scholar] [CrossRef]
- Vaalamo, M.; Leivo, T.; Saarialho-Kere, U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1,-2,-3, and-4) in normal and aberrant wound healing. Hum. Pathol. 1999, 30, 795–802. [Google Scholar] [CrossRef]
- Knuutinen, A.; Kokkonen, N.; Risteli, J.; Vähäkangas, K.; Kallioinen, M.; Salo, T.; Sorsa, T.; Oikarinen, A. Smoking affects collagen synthesis and extracellular matrix turnover in human skin. Br. J. Dermatol. 2002, 146, 588–594. [Google Scholar] [CrossRef]
- Brazil, D.P.; Park, J.; Hemmings, B.A. PKB binding proteins: Getting in on the Akt. Cell 2002, 111, 293–303. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, J.-H.; Kim, H.J.; Park, M.K.; Huh, J.W.; Ro, J.Y.; Oh, Y.-M.; Lee, S.-D.; Lee, Y.-S. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L891–L908. [Google Scholar] [CrossRef]
- Zimolag, E.; Borowczyk-Michalowska, J.; Kedracka-Krok, S.; Skupien-Rabian, B.; Karnas, E.; Lasota, S.; Sroka, J.; Drukala, J.; Madeja, Z. Electric field as a potential directional cue in homing of bone marrow-derived mesenchymal stem cells to cutaneous wounds. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 267–279. [Google Scholar] [CrossRef]
- Wang, X.-F.; Li, M.-L.; Fang, Q.-Q.; Zhao, W.-Y.; Lou, D.; Hu, Y.-Y.; Chen, J.; Wang, X.-Z.; Tan, W.-Q. Flexible electrical stimulation device with Chitosan-Vaseline® dressing accelerates wound healing in diabetes. Bioact. Mater. 2021, 6, 230–243. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Du, J.; Reid, B.; Deng, X.; Liu, Z.; Zong, Z.; Wang, H.; Yao, B.; Yang, C. Electric fields guide migration of epidermal stem cells and promote skin wound healing. Wound Repair Regen. 2012, 20, 840–851. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Prahm, C.; Chen, Y.; Ehnert, S.; Rinderknecht, H.; McCaig, C.D.; Nussler, A.K.; Kolbenschlag, J. Microcurrent Reverses Cigarette Smoke-Induced Angiogenesis Impairment in Human Keratinocytes In Vitro. Bioengineering 2022, 9, 445. https://doi.org/10.3390/bioengineering9090445
Lu C, Prahm C, Chen Y, Ehnert S, Rinderknecht H, McCaig CD, Nussler AK, Kolbenschlag J. Microcurrent Reverses Cigarette Smoke-Induced Angiogenesis Impairment in Human Keratinocytes In Vitro. Bioengineering. 2022; 9(9):445. https://doi.org/10.3390/bioengineering9090445
Chicago/Turabian StyleLu, Chao, Cosima Prahm, Yangmengfan Chen, Sabrina Ehnert, Helen Rinderknecht, Colin D. McCaig, Andreas K. Nussler, and Jonas Kolbenschlag. 2022. "Microcurrent Reverses Cigarette Smoke-Induced Angiogenesis Impairment in Human Keratinocytes In Vitro" Bioengineering 9, no. 9: 445. https://doi.org/10.3390/bioengineering9090445