Anti-Tumor Effect of Parasitic Protozoans
Abstract
:1. Introduction
2. Tumor Therapy with the Injection of Parasite
3. Antitumor Effect of Parasitic Products
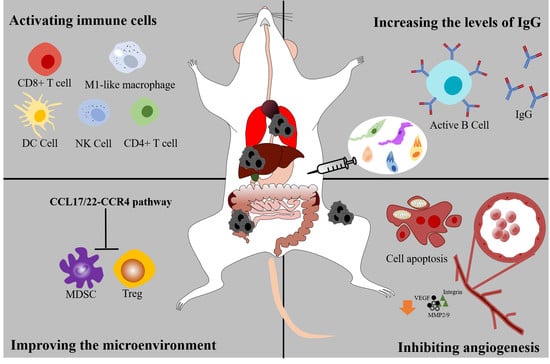
4. Activating the Cellular Immune System
4.1. Immune Cells
4.2. Cytokines
5. Activating the Humoral Immunity System
6. Suppressing the Angiogenesis and Tumor Metastasis
7. Improving the Microenvironment
8. Conclusions and Future Directions
| Parasite | Cancer | Mechanism of Action | Reference |
|---|---|---|---|
| Leishmania spp. | breast cancer; HPV-associated tumors | Activation of CD4+ and CD8+ T cells, macrophages in spleen or NK cell; Induction of proinflammatory cytokines that help the generation of protective Th1 responses; Increasing the levels of IgG2a | [6,81] |
| Neospora caninum | murine thymoma EG7 | Activation of NK cell- and CD8-T cell-dependent protective antitumor response; IFN-γ secretion in tumor microenvironment | [7] |
| Eimeria spp. | Sarcoma tumor S180 | EA upregulates inflammatory modulators MCP-1, IL-6, IL-12, IFN-γ, and TNF-α | [26] |
| Toxoplasma gondii | Melanoma; Lewis lung carcinoma; Ehrlich’s adenocarcinoma; Pancreatic ductal adenocarcinoma; ovarian carcinoma | Secreted proteins activate antitumor immune responses involving CD4+ and CD8+ T cells, IL-12, IFN-γ and TNF-α or activation of NK cell; Increase the levels of IgG1 and IgG2a; Suppressed the levels of angiogenic factors (VEGF, integrin, MMP2, and MMP9) | [8,12,23,60,95] |
| Trypanosoma cruzi | mammary cancer; colon cancer; Melanoma; Ehrlich’s adenocarcinoma | Calreticulin inhibits vascular endothelial growth factor (VEGF)-induced cell proliferation and induces cell apoptosis; activation of CD4+ and CD8+ T cells and macrophages and DC | [30,101,102,103] |
| Plasmodium spp. | non-Hodgkin’s lymphoma (Karpas299) and prostate cancer (PC-3); Lewis lung cancer; hepatocellular carcinoma; breast cancer | rVAR2 binds with the distinct oncofetal chondroitin sulfate that makes rVAR2 a potential ideal carrier for anti-cancer drug delivery; Activation of NK cell, DC, CD8+ T cell; Suppressed the levels of angiogenic factors (VEGF, MMP9, IGF); Reduce the numbers of MDSC and Treg through CCL17/22-CCR4 pathway | [18,104,105,106] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Roskin, G. Toxin therapy of experimental cancer; the influence of protozoan infections upon transplanted cancer. Cancer Res. 1946, 6, 363–365. [Google Scholar] [PubMed]
- Hauschka, T.S.; Goodwin, M.B. Trypanosoma cruzi Endotoxin (KR) in the Treatment of Malignant Mouse Tumors. Science 1948, 107, 600–602. [Google Scholar] [CrossRef]
- Ramirez-Toloza, G.; Abello, P.; Ferreira, A. Is the Antitumor Property of Trypanosoma cruzi Infection Mediated by Its Calreticulin? Front. Immunol. 2016, 7, 268. [Google Scholar] [CrossRef]
- Caner, A.; Sadiqova, A.; Erdogan, A.; Namlises, D.; Nalbantsoy, A.; Oltulu, F.; Toz, S.; Yigitturk, G.; Ozkok, E.; Gunduz, C.; et al. Targeting of antitumor immune responses with live-attenuated Leishmania strains in breast cancer model. Breast Cancer 2020, 27, 1082–1095. [Google Scholar] [CrossRef]
- Lantier, L.; Poupée-Beaugé, A.; Di Tommaso, A.; Ducournau, C.; Epardaud, M.; Lakhrif, Z.; Germon, S.; Debierre-Grockiego, F.; Mévélec, M.-N.; Battistoni, A.; et al. Neospora caninum: A new class of biopharmaceuticals in the therapeutic arsenal against cancer. J. Immunother. Cancer 2020, 8, e001242. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Elsheikha, H.M.; Wang, J.H.; Fang, S.; He, J.J.; Zhu, X.Q.; Chen, J. Synergy between Toxoplasma gondii type I DeltaGRA17 immunotherapy and PD-L1 checkpoint inhibition triggers the regression of targeted and distal tumors. J. Immunother. Cancer 2021, 9, e002970. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dey, R.; Dagur, P.K.; Kruhlak, M.; Ismail, N.; Debrabant, A.; Joshi, A.B.; Akue, A.; Kukuruga, M.; Takeda, K.; et al. Genetically Modified Live Attenuated Leishmania donovani Parasites Induce Innate Immunity through Classical Activation of Macrophages That Direct the Th1 Response in Mice. Infect. Immun. 2015, 83, 3800–3815. [Google Scholar] [CrossRef]
- Kochanowsky, J.A.; Koshy, A.A. Toxoplasma gondii. Curr. Biol. 2018, 28, R770–R771. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kobayashi, A. Antitumor effect of intralesional injection with formalin-fixed toxoplasma gondii organisms on lewis lung carcinoma in toxoplasma-infected mice. Cancer Lett. 1985, 25, 247–254. [Google Scholar] [CrossRef]
- Hafez, E.N.; Moawed, F.S.M.; Abdel-Hamid, G.R.; Elbakary, N.M. Gamma Radiation-Attenuated Toxoplasma gondii Provokes Apoptosis in Ehrlich Ascites Carcinoma-Bearing Mice Generating Long-Lasting Immunity. Technol. Cancer Res. Treat. 2020, 19, 1533033820926593. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Xia, N.; Zhou, T.; Shen, B. Antitumor effects of a Toxoplasma mutant lacking lactate dehydrogenases. Parasitol. Res. 2021, 120, 3335–3339. [Google Scholar] [CrossRef]
- Baird, J.R.; Fox, B.A.; Sanders, K.L.; Lizotte, P.H.; Cubillos-Ruiz, J.R.; Scarlett, U.K.; Rutkowski, M.R.; Conejo-Garcia, J.R.; Fiering, S.; Bzik, D.J. Avirulent Toxoplasma gondii Generates Therapeutic Antitumor Immunity by Reversing Immunosuppression in the Ovarian Cancer Microenvironment. Cancer Res. 2013, 73, 3842–3851. [Google Scholar] [CrossRef]
- Sugahara, K.; Mikami, T.; Uyama, T.; Mizuguchi, S.; Nomura, K.; Kitagawa, H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003, 13, 612–620. [Google Scholar] [CrossRef]
- Shao, C.; Shi, X.; White, M.; Huang, Y.; Hartshorn, K.; Zaia, J. Comparative glycomics of leukocyte glycosaminoglycans. FEBS J. 2013, 280, 2447–2461. [Google Scholar] [CrossRef]
- Clausen, T.M.; Pereira, M.A.; Al Nakouzi, N.; Oo, H.Z.; Agerbæk, M.Ø.; Lee, S.; Ørum-Madsen, M.S.; Kristensen, A.R.; El-Naggar, A.; Grandgenett, P.M.; et al. Oncofetal Chondroitin Sulfate Glycosaminoglycans Are Key Players in Integrin Signaling and Tumor Cell Motility. Mol. Cancer Res. 2016, 14, 1288–1299. [Google Scholar] [CrossRef]
- Salanti, A.; Clausen, T.M.; Agerbæk, M.Ø.; Al Nakouzi, N.; Dahlbäck, M.; Oo, H.Z.; Lee, S.; Gustavsson, T.; Rich, J.R.; Hedberg, B.J.; et al. Targeting Human Cancer by a Glycosaminoglycan Binding Malaria Protein. Cancer Cell 2015, 28, 500–514. [Google Scholar] [CrossRef]
- Clausen, T.M.; Christoffersen, S.; Dahlbäck, M.; Langkilde, A.E.; Jensen, K.E.; Resende, M.; Agerbæk, M.; Andersen, D.; Berisha, B.; Ditlev, S.B.; et al. Structural and Functional Insight into How the Plasmodium falciparum VAR2CSA Protein Mediates Binding to Chondroitin Sulfate A in Placental Malaria. J. Biol. Chem. 2012, 287, 23332–23345. [Google Scholar] [CrossRef]
- Agerbaek, M.O.; Bang-Christensen, S.R.; Yang, M.H.; Clausen, T.M.; Pereira, M.A.; Sharma, S.; Ditlev, S.B.; Nielsen, M.A.; Choudhary, S.; Gustavsson, T.; et al. The VAR2CSA malaria protein efficiently retrieves circulating tumor cells in an EpCAM-independent manner. Nat. Commun. 2018, 9, 3279. [Google Scholar] [CrossRef]
- Dlugonska, H. Toxoplasma Rhoptries: Unique Secretory Organelles and Source of Promising Vaccine Proteins for Immunoprevention of Toxoplasmosis. J. Biomed. Biotechnol. 2008, 2008, 632424. [Google Scholar] [CrossRef]
- Panas, M.W.; Boothroyd, J.C. Seizing control: How dense granule effector proteins enable Toxoplasma to take charge. Mol. Microbiol. 2021, 115, 466–477. [Google Scholar] [CrossRef]
- Fox, B.A.; Sanders, K.L.; Rommereim, L.M.; Guevara, R.B.; Bzik, D.J. Secretion of Rhoptry and Dense Granule Effector Proteins by Nonreplicating Toxoplasma gondii Uracil Auxotrophs Controls the Development of Antitumor Immunity. PLoS Genet. 2016, 12, e1006189. [Google Scholar] [CrossRef]
- Wang, P.; Li, S.; Zhao, Y.; Zhang, B.; Li, Y.; Liu, S.; Du, H.; Cao, L.; Ou, M.; Ye, X.; et al. The GRA15 protein from Toxoplasma gondii enhances host defense responses by activating the interferon stimulator STING. J. Biol. Chem. 2019, 294, 16494–16508. [Google Scholar] [CrossRef]
- Seo, S.H.; Kim, S.G.; Shin, J.H.; Ham, D.W.; Shin, E.H. Toxoplasma GRA16 Inhibits NF-kappaB Activation through PP2A-B55 Upregulation in Non-Small-Cell Lung Carcinoma Cells. Int. J. Mol. Sci. 2020, 21, 6642. [Google Scholar] [CrossRef]
- Rosenberg, B.; Juckett, D.A.; Aylsworth, C.F.; Dimitrov, N.V.; Ho, S.-C.; Judge, J.W.; Kessel, S.; Quensen, J.; Wong, K.-P.H.; Zlatkin, I.; et al. Protein from intestinal Eimeria protozoan stimulates IL-12 release from dendritic cells, exhibits antitumor properties in vivo and is correlated with low intestinal tumorigenicity. Int. J. Cancer 2004, 114, 756–765. [Google Scholar] [CrossRef]
- Junqueira, C.; Santos, L.I.; Galvão-Filho, B.; Teixeira, S.M.; Rodrigues, F.G.; DaRocha, W.D.; Chiari, E.; Jungbluth, A.A.; Ritter, G.; Gnjatic, S.; et al. Trypanosoma cruzi as an effective cancer antigen delivery vector. Proc. Natl. Acad. Sci. USA 2011, 108, 19695–19700. [Google Scholar] [CrossRef]
- Ramírez, G.; Valck, C.; Aguilar, L.; Kemmerling, U.; López-Muñoz, R.; Cabrera, G.; Morello, A.; Ferreira, J.; Maya, J.D.; Galanti, N.; et al. Roles of Trypanosoma cruzi calreticulin in parasite–host interactions and in tumor growth. Mol. Immunol. 2012, 52, 133–140. [Google Scholar] [CrossRef]
- Molina, M.C.; Ferreira, V.; Valck, C.; Aguilar, L.; Orellana, J.; Rojas, A.; Ramirez, G.; Billetta, R.; Schwaeble, W.; Lemus, D.; et al. An in vivo role for Trypanosoma cruzi calreticulin in antiangiogenesis. Mol. Biochem. Parasitol. 2005, 140, 133–140. [Google Scholar] [CrossRef]
- López, N.C.; Valck, C.; Ramírez, G.; Rodríguez, M.; Ribeiro, C.; Orellana, J.; Maldonado, I.; Albini, A.; Anacona, D.; Lemus, D.; et al. Antiangiogenic and Antitumor Effects of Trypanosoma cruzi Calreticulin. PLoS Neglected Trop. Dis. 2010, 4, e730. [Google Scholar] [CrossRef]
- Shah, M.A.K.A.S.A.A.A.; Kamal, M.A.; Akhtar, S. Tumor Angiogenesis and VEGFR-2: Mechanism, Pathways and Current Biological Therapeutic Interventions. Curr. Drug Metab. 2021, 22, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor Angiogenesis: Current Challenges and Therapeutic Opportunities (Review). Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Li, W.; Li, H.; Sun, G. Vasostatin Inhibits VEGF-Induced Endothelial Cell Proliferation, Tube Formation and Induces Cell Apoptosis under Oxygen Deprivation. Int. J. Mol. Sci. 2014, 15, 6019–6030. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Gabrilovich, D.; Pisarev, V. Tumor escape from immune response: Mechanisms and targets of activity. Curr. Drug Targets 2003, 4, 525–536. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688, Correction in 2018, 18, 726. [Google Scholar] [CrossRef]
- Hammer, Q.; Rückert, T.; Borst, E.M.; Dunst, J.; Haubner, A.; Durek, P.; Heinrich, F.; Gasparoni, G.; Babic, M.; Tomic, A.; et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat. Immunol. 2018, 19, 453–463. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK cells and ILCs in tumor immunotherapy. Mol. Asp. Med. 2020, 80, 100870. [Google Scholar] [CrossRef]
- Chen, L.; He, Z.; Qin, L.; Li, Q.; Shi, X.; Zhao, S.; Chen, L.; Zhong, N.; Chen, X. Antitumor Effect of Malaria Parasite Infection in a Murine Lewis Lung Cancer Model through Induction of Innate and Adaptive Immunity. PLoS ONE 2011, 6, e24407. [Google Scholar] [CrossRef]
- Korbel, D.S.; Newman, K.C.; Almeida, C.R.; Davis, D.M.; Riley, E.M. Heterogeneous Human NK Cell Responses to Plasmodium falciparum-Infected Erythrocytes. J. Immunol. 2005, 175, 7466–7473. [Google Scholar] [CrossRef]
- Chen, X.; Qin, L.; Hu, W.; Adah, D. The mechanisms of action of Plasmodium infection against cancer. Cell Commun. Signal. 2021, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, M.X.H.; Vargas-Inchaustegui, D.A.; Xin, L.; Soong, L. Role of Natural Killer Cells in Modulating Dendritic Cell Responses to Leishmania amazonensis Infection. Infect. Immun. 2008, 76, 5100–5109. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.R.; Byrne, K.T.; Lizotte, P.H.; Toraya-Brown, S.; Scarlett, U.K.; Alexander, M.P.; Sheen, M.R.; Fox, B.A.; Bzik, D.J.; Bosenberg, M.; et al. Immune-Mediated Regression of Established B16F10 Melanoma by Intratumoral Injection of Attenuated Toxoplasma gondii Protects against Rechallenge. J. Immunol. 2012, 190, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Patel, S.; Wang, Q.; Andhey, P.; Zaitsev, K.; Porter, S.; Hershey, M.; Bern, M.; Plougastel-Douglas, B.; Collins, P.; et al. Toxoplasma gondii infection drives conversion of NK cells into ILC1-like cells. ELife 2019, 8, e47605. [Google Scholar] [CrossRef]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawam, J.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- Kamiya, T.; Chang, Y.-H.; Campana, D. Expanded and Activated Natural Killer Cells for Immunotherapy of Hepatocellular Carcinoma. Cancer Immunol. Res. 2016, 4, 574–581. [Google Scholar] [CrossRef]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2017, 32, 520–531. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Nishikawa, H. Roles of regulatory T cells in cancer immunity. Int. Immunol. 2016, 28, 401–409. [Google Scholar] [CrossRef]
- Choi, Y.; Shi, Y.; Haymaker, C.L.; Naing, A.; Ciliberto, G.; Hajjar, J. T-cell agonists in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000966. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Okada, M.; Shimizu, K.; Fujii, S.-I. Identification of Neoantigens in Cancer Cells as Targets for Immunotherapy. Int. J. Mol. Sci. 2022, 23, 2594. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Baitsch, L.; Baumgaertner, P.; Devêvre, E.; Raghav, S.K.; Legat, A.; Barba, L.; Wieckowski, S.; Bouzourene, H.; Deplancke, B.; Romero, P.; et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Investig. 2011, 121, 2350–2360. [Google Scholar] [CrossRef]
- Dey, R.; Dagur, P.K.; Selvapandiyan, A.; McCoy, J.P.; Salotra, P.; Duncan, R.; Nakhasi, H.L. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J. Immunol. 2013, 190, 2138–2149. [Google Scholar] [CrossRef]
- Dey, R.; Natarajan, G.; Bhattacharya, P.; Cummings, H.; Dagur, P.K.; Terrazas, C.; Selvapandiyan, A.; McCoy, J.P.; Duncan, R.; Satoskar, A.R.; et al. Characterization of Cross-Protection by Genetically Modified Live-Attenuated Leishmania donovani Parasites against Leishmania mexicana. J. Immunol. 2014, 193, 3513–3527. [Google Scholar] [CrossRef]
- Sanders, K.L.; Fox, B.A.; Bzik, D.J. Attenuated Toxoplasma gondii therapy of disseminated pancreatic cancer generates long-lasting immunity to pancreatic cancer. Oncoimmunology 2016, 5, e1104447. [Google Scholar] [CrossRef]
- Verneau, J.; Sautés-Fridman, C.; Sun, C.-M. Dendritic cells in the tumor microenvironment: Prognostic and theranostic impact. Semin. Immunol. 2020, 48, 101410. [Google Scholar] [CrossRef]
- Mildner, A.; Jung, S. Development and Function of Dendritic Cell Subsets. Immunity 2014, 40, 642–656. [Google Scholar] [CrossRef]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- De Veer, M.J.; Curtis, J.M.; Baldwin, T.M.; DiDonato, J.A.; Sexton, A.; McConville, M.J.; Handman, E.; Schofield, L. MyD88 is essential for clearance ofLeishmania major: Possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 2003, 33, 2822–2831. [Google Scholar] [CrossRef]
- Campos, M.A.S.; Almeida, I.C.; Takeuchi, O.; Akira, S.; Valente, E.P.; Procópio, D.O.; Travassos, L.R.; Smith, J.A.; Golenbock, D.T.; Gazzinelli, R.T. Activation of Toll-Like Receptor-2 by Glycosylphosphatidylinositol Anchors from a Protozoan Parasite. J. Immunol. 2001, 167, 416–423. [Google Scholar] [CrossRef]
- Krishnegowda, G.; Hajjar, A.M.; Zhu, J.; Douglass, E.J.; Uematsu, S.; Akira, S.; Woods, A.S.; Gowda, D.C. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: Cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J. Biol. Chem. 2005, 280, 8606–8616. [Google Scholar] [CrossRef]
- Debierre-Grockiego, F.; Azzouz, N.; Schmidt, J.; Dubremetz, J.F.; Geyer, H.; Geyer, R.; Weingart, R.; Schmidt, R.R.; Schwarz, R.T. Roles of glycosylphosphatidylinositols of Toxoplasma gondii. Induction of tumor necrosis factor-alpha production in macrophages. J. Biol. Chem. 2003, 278, 32987–32993. [Google Scholar] [CrossRef]
- Yarovinsky, F.; Zhang, D.; Andersen, J.F.; Bannenberg, G.L.; Serhan, C.N.; Hayden, M.S.; Hieny, S.; Sutterwala, F.S.; Flavell, R.A.; Ghosh, S.; et al. TLR11 Activation of Dendritic Cells by a Protozoan Profilin-Like Protein. Science 2005, 308, 1626–1629. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-gamma in tumor progression and regression: A review. Biomark Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. Impact of IL-12 in Cancer. Curr. Cancer Drug Targets 2017, 17, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Batchu, R.B.; Gruzdyn, O.V.; Kolli, B.K.; Dachepalli, R.; Umar, P.S.; Rai, S.K.; Singh, N.; Tavva, P.S.; Weaver, D.W.; Gruber, S.A. IL-10 Signaling in the Tumor Microenvironment of Ovarian Cancer. Adv. Exp. Med. Biol. 2021, 1290, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Zhou, Y.; Zhou, P.; Yan, Q.; Chen, X.; Ding, S.; Zhu, F. CKLF1 Enhances Inflammation-Mediated Carcinogenesis and Prevents Doxorubicin-Induced Apoptosis via IL6/STAT3 Signaling in HCC. Clin. Cancer Res. 2019, 25, 4141–4154. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, R.; Cai, T.; Chen, Z.; Lan, M.; Zou, T.; Wang, B.; Wang, Q.; Zhao, Y.; Cai, Y. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int. Immunopharmacol. 2020, 88, 106939. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-O.; Jung, S.-S.; Kim, S.-Y.; Kim, T.Y.; Shin, D.-W.; Lee, J.-H.; Lee, Y.-H. Inhibition of Lewis Lung Carcinoma Growth by Toxoplasma gondii through Induction of Th1 Immune Responses and Inhibition of Angiogenesis. J. Korean Med Sci. 2007, 22, S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.N.; Youssef, H.; El-Kabany, H.A. Vaccination with gamma radiation-attenuated Toxoplasma gondii protects against ovarian infiltration in mice-bearing Ehrlich ascites carcinoma. Int. J. Radiat. Biol. 2020, 96, 814–822. [Google Scholar] [CrossRef]
- Astaneh, M.; Dashti, S.; Esfahani, Z.T. Humoral immune responses against cancer-testis antigens in human malignancies. Hum. Antibodies 2019, 27, 237–240. [Google Scholar] [CrossRef]
- Rudnick, S.I.; Lou, J.; Shaller, C.C.; Tang, Y.; Klein-Szanto, A.J.; Weiner, L.M.; Marks, J.D.; Adams, G.P. Influence of Affinity and Antigen Internalization on the Uptake and Penetration of Anti-HER2 Antibodies in Solid Tumors. Cancer Res. 2011, 71, 2250–2259. [Google Scholar] [CrossRef]
- Salehi, M.; Taheri, T.; Mohit, E.; Zahedifard, F.; Seyed, N.; Taslimi, Y.; Sattari, M.; Bolhassani, A.; Rafati, S. Recombinant Leishmania tarentolae encoding the HPV type 16 E7 gene in tumor mice model. Immunotherapy 2012, 4, 1107–1120. [Google Scholar] [CrossRef]
- Zenina, A.V.; Kravtsov, E.G.; Tsetsegsaikhan, B.; Yashina, N.V.; Dalin, M.V.; Karpenko, L.P.; Sheklakova, L.A.; Kallinikova, V.D. The study of immunological component in antitumor effect of Trypanosoma cruzi. Bull. Exp. Biol. Med. 2008, 145, 352–354. [Google Scholar] [CrossRef]
- Tas, S.W.; Maracle, C.; Balogh, E.; Szekanecz, Z. Targeting of proangiogenic signalling pathways in chronic inflammation. Nat. Rev. Rheumatol. 2015, 12, 111–122. [Google Scholar] [CrossRef]
- Wang, B.; Li, Q.; Wang, J.; Zhao, S.; Nashun, B.; Qin, L.; Chen, X. Plasmodium infection inhibits tumor angiogenesis through effects on tumor-associated macrophages in a murine implanted hepatoma model. Cell Commun. Signal. 2020, 18, 157. [Google Scholar] [CrossRef]
- Shieh, Y.-S.; Hung, Y.-J.; Hsieh, C.-B.; Chen, J.-S.; Chou, K.-C.; Liu, S.-Y. Tumor-Associated Macrophage Correlated with Angiogenesis and Progression of Mucoepidermoid Carcinoma of Salivary Glands. Ann. Surg. Oncol. 2008, 16, 751–760. [Google Scholar] [CrossRef]
- Ferreira, V.; Valck, C.; Sánchez, G.; Gingras, A.; Tzima, S.; Molina, M.C.; Sim, R.; Schwaeble, W.; Ferreira, A. The Classical Activation Pathway of the Human Complement System Is Specifically Inhibited by Calreticulin from Trypanosoma cruzi. J. Immunol. 2004, 172, 3042–3050. [Google Scholar] [CrossRef]
- Ramírez, G.; Valck, C.; Molina, M.C.; Ribeiro, C.H.; López, N.; Sánchez, G.; Ferreira, V.P.; Billetta, R.; Aguilar, L.; Maldonado, I.; et al. Trypanosoma cruzi calreticulin: A novel virulence factor that binds complement C1 on the parasite surface and promotes infectivity. Immunobiology 2011, 216, 265–273. [Google Scholar] [CrossRef]
- Pyo, K.-H.; Jung, B.-K.; Chai, J.-Y.; Shin, E.-H. Suppressed CD31 Expression in Sarcoma-180 Tumors after Injection with Toxoplasma gondii Lysate Antigen in BALB/c Mice. Korean J. Parasitol. 2010, 48, 171–174. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Bayne, L.J. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr. Opin. Immunol. 2013, 25, 200–205. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2016, 27, 109–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Velez-Delgado, A.; Mathew, E.; Li, D.; Mendez, F.M.; Flannagan, K.; Rhim, A.D.; Simeone, D.M.; Beatty, G.L.; Pasca di Magliano, M. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 2017, 66, 124–136. [Google Scholar] [CrossRef]
- Wang, X.; Lang, M.; Zhao, T.; Feng, X.; Zheng, C.; Huang, C.; Hao, J.; Dong, J.; Luo, L.; Li, X.; et al. Cancer-FOXP3 directly activated CCL5 to recruit FOXP3+Treg cells in pancreatic ductal adenocarcinoma. Oncogene 2016, 36, 3048–3058. [Google Scholar] [CrossRef]
- Adah, D.; Yang, Y.; Liu, Q.; Gadidasu, K.; Tao, Z.; Yu, S.; Dai, L.; Li, X.; Zhao, S.; Qin, L.; et al. Plasmodium infection inhibits the expansion and activation of MDSCs and Tregs in the tumor microenvironment in a murine Lewis lung cancer model. Cell Commun. Signal. 2019, 17, 32. [Google Scholar] [CrossRef]
- Payne, S.N.; Emmerich, P.B.; Davis, N.M.; Deming, D.A.; Knoll, L.J. Novel Murine Pancreatic Tumor Model Demonstrates Immunotherapeutic Control of Tumor Progression by a Toxoplasma gondii Protein. Infect. Immun. 2021, 89, e00508-21. [Google Scholar] [CrossRef]
- Bahwal, S.A.; Chen, J.J.; Lilin, E.; Hao, T.; Chen, J.; Carruthers, V.B.; Lai, J.; Zhou, X. Attenuated Toxoplasma gondii enhances the antitumor efficacy of anti-PD1 antibody by altering the tumor microenvironment in a pancreatic cancer mouse model. J. Cancer Res. Clin. Oncol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Hatter, J.A.; Kouche, Y.M.; Melchor, S.J.; Ng, K.; Bouley, D.M.; Boothroyd, J.C.; Ewald, S.E. Toxoplasma gondii infection triggers chronic cachexia and sustained commensal dysbiosis in mice. PLoS ONE 2018, 13, e0204895. [Google Scholar] [CrossRef]
- Truyens, C.; Torrico, F.; Angelo-Barrios, A.; Lucas, R.; Heremans, H.; De Baetselier, P.; Carlier, Y. The cachexia associated with Trypanosoma cruzi acute infection in mice is attenuated by anti-TNF-alpha, but not by anti-IL-6 or anti-IFN-gamma antibodies. Parasite Immunol. 1995, 17, 561–568. [Google Scholar] [CrossRef]
- Lamb, T.; Brown, D.E.; Potocnik, A.J.; Langhorne, J. Insights into the immunopathogenesis of malaria using mouse models. Expert Rev. Mol. Med. 2006, 8, 1–22. [Google Scholar] [CrossRef]
- Herremans, K.M.; Riner, A.N.; Cameron, M.E.; Trevino, J.G. The Microbiota and Cancer Cachexia. Int. J. Mol. Sci. 2019, 20, 6267. [Google Scholar] [CrossRef]
- Ubillos, L.; Freire, T.; Berriel, E.; Chiribao, M.L.; Chiale, C.; Festari, M.F.; Medeiros, A.; Mazal, D.; Rondan, M.; Bollati-Fogolin, M.; et al. Trypanosoma cruzi extracts elicit protective immune response against chemically induced colon and mammary cancers. Int. J. Cancer 2016, 138, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, L.A.A.; Lobos-Gonzalez, L.; Rosas, C.; Vallejos, G.; Falcón, C.; Sosoniuk, E.; Coddou, F.; Leyton, L.; Lemus, D.; Quest, A.G.; et al. Human Survivin and Trypanosoma cruzi Calreticulin Act in Synergy against a Murine Melanoma In Vivo. PLoS ONE 2014, 9, e95457. [Google Scholar] [CrossRef]
- Atayde, V.D.; Jasiulionis, M.G.; Cortez, M.; Yoshida, N. A recombinant protein based on Trypanosoma cruzi surface molecule gp82 induces apoptotic cell death in melanoma cells. Melanoma Res. 2008, 18, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, M.; Qin, L.; Kang, Z.; Adah, D.; Tao, Z.; Li, X.; Dai, L.; Zhao, S.; Chen, X.; et al. Plasmodium infection inhibits triple negative 4T1 breast cancer potentially through induction of CD8+ T cell-mediated antitumor responses in mice. Biomed. Pharmacother. 2021, 138, 111406. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, X.; Tao, Z.; Ma, M.; Adah, D.; Li, X.; Dai, L.; Ding, W.; Fanuel, S.; Zhao, S.; et al. Plasmodium infection prevents recurrence and metastasis of hepatocellular carcinoma possibly via inhibition of the epithelial-mesenchymal transition. Mol. Med. Rep. 2021, 23, 418. [Google Scholar] [CrossRef]
- Zhou, D.; Zheng, H.; Liu, Q.; Lu, X.; Deng, X.; Jiang, L.; Hou, B.; Fu, Y.; Zhu, F.; Ding, Y.; et al. Attenuated plasmodium sporozoite expressing MAGE-A3 induces antigen-specific CD8+ T cell response against lung cancer in mice. Cancer Biol. Med. 2019, 16, 288–298. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Wu, S.; Jin, Z.; Zheng, B.; Hu, Y.; He, K.; Lu, S.; Zhuo, X. Anti-Tumor Effect of Parasitic Protozoans. Bioengineering 2022, 9, 395. https://doi.org/10.3390/bioengineering9080395
Ding H, Wu S, Jin Z, Zheng B, Hu Y, He K, Lu S, Zhuo X. Anti-Tumor Effect of Parasitic Protozoans. Bioengineering. 2022; 9(8):395. https://doi.org/10.3390/bioengineering9080395
Chicago/Turabian StyleDing, Haojie, Songrui Wu, Zi Jin, Bin Zheng, Yuan Hu, Ke He, Shaohong Lu, and Xunhui Zhuo. 2022. "Anti-Tumor Effect of Parasitic Protozoans" Bioengineering 9, no. 8: 395. https://doi.org/10.3390/bioengineering9080395