Bone Formation and Maintenance in Oral Surgery: The Decisive Role of the Immune System—A Narrative Review of Mechanisms and Solutions
Abstract
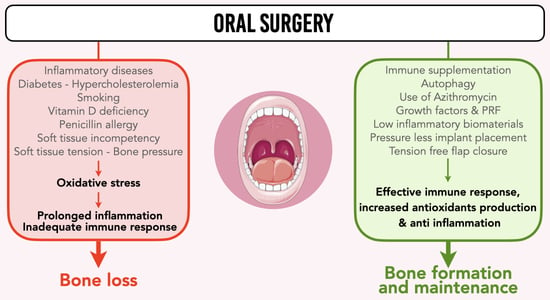
:1. Introduction
2. Osteoimmunology
2.1. Immune System and Immunity
- a.
- The innate immune system is the first line of defense. It is the first structure of the body to detect pathogens such as viruses, bacteria, parasites and toxins, or to sense wounds and trauma. Besides playing this role, the innate immune system activates corresponding cells to attack and knock down microbes and initiate tissue healing and remodeling. Inflammation is the main mechanism of innate immunity, and its effector cells are phagocytic cells (granulocytes, monocytes, macrophages, dendritic cells), epithelial and endothelial cells, natural killer (NK) cells, innate lymphoid cells (ILC) and platelets [20]. After this first level of immune response has been activated, mediators inform and modulate the adaptive immune response.
- b.
- The adaptive (or acquired) immune system is the second and specific line of defense and is developed after exposure to microbes or their released chemicals. Lymphocytes are the main cells of acquired immunity. T-lymphocytes are in control of cell-mediated immunity, while B-lymphocytes handle humoral immunity with antibodies. Excessive levels of specific immune responses lead to allergic reactions or the development of autoimmune diseases [21].
2.2. Bone Repair Process and Inflammation
2.3. Influence of Immune System on Alveolar Bone
3. Oxidation and Bone
3.1. Oxidative Stress
3.2. Oxidative Stress and Bone
4. Origins of Oxidative Stress and Immune Deficiency in Oral Surgery
4.1. Biological and Metabolic Effects
4.2. Penicillin Allergy
4.3. Chronic Inflammation
4.4. Chronic Hypoxia
5. Review of Solutions to Improve Immune Response and Reduce Levels of Oxidative Stress
5.1. Systemic Improvements in Immune Response and Anti-Inflammation Strategies
- a.
- Antioxidation by nutraceuticals
- b.
- Antioxidation by reduction in levels of serum LDL cholesterol
- c.
- Autophagy
- d.
- Use of immunomodulatory antibiotics
5.2. Local Improvement in Immune Response and Anti-Inflammation Strategies
- a.
- Growth factors and Platelet-Rich Fibrin
- b.
- Use of low-inflammatory biomaterials
- c.
- Pressure-less implant placement
- d.
- Sutures and tension-free flap closure
- e.
- Topical azithromycin
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Su, L.; Zheng, J.; Wang, Y.; Zhang, W.; Hu, D. Emerging progress on the mechanism and technology in wound repair. Biomed Pharmacother. 2019, 117, 109191. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Crivellato, E. Immune cells and angiogenesis. J. Cell. Mol. Med. 2009, 13, 2822–2833. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Goto, M.; Mochizuki, S.I.; Tsuda, E.; Morinaga, T.; Udagawa, N.; et al. A novel molecular mechanism modulating osteoclast differentiation and function. Bone 1999, 25, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol. Rev. 2017, 97, 1295–1349. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress; Academic Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Brunetti, G.; D’Amelio, P.; Mori, G.; Faienza, M.F. Editorial: Updates on Osteoimmunology: What’s New on the Crosstalk Between Bone and Immune Cells. Front. Endocrinol. 2020, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Mikami, R.; Mizutani, K.; Takeda, K.; Kominato, H.; Kido, D.; Ikeda, Y.; Buranasin, P.; Nakagawa, K.; Takemura, S.; et al. Impaired dental implant osseointegration in rat with streptozotocin-induced diabetes. J. Periodontal. Res. 2022, 57, 412–424. [Google Scholar] [CrossRef]
- Simonis, P.; Dufour, T.; Tenenbaum, H. Long-term implant survival and success: A 10-16-year follow-up of non-submerged dental implants. Clin. Oral. Implant. Res. 2010, 21, 772–777. [Google Scholar] [CrossRef]
- Lee, C.T.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Alsabeeha, N.H.; Faggion, C.M., Jr.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Galindo-Moreno, P.; Monje, A.; Radovanovic, S.; Wang, H.L.; Cochran, D.; Sculean, A.; Canullo, L. How frequently does peri-implantitis occur? A systematic review and meta-analysis. Clin. Oral. Investig. 2018, 22, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkane, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal. Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Chrcanovic, B.; Mölne, J.; Wennerberg, A. Foreign body reactions, marginal bone loss and allergies in relation to titanium implants. Eur. J. Oral. Implantol. 2018, 11 (Suppl. S1), S37–S46. [Google Scholar] [PubMed]
- Qian, J.; Wennerberg, A.; Albrektsson, T. Reasons for marginal bone loss around oral implants. Clin. Implant Dent. Relat. Res. 2012, 14, 792–807. [Google Scholar] [CrossRef] [PubMed]
- Mori, G.; D’Amelio, P.; Faccio, R.; Brunetti, G. The Interplay between the bone and the immune system. Clin. Dev. Immunol. 2013, 720504. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef]
- Guder, C.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Osteoimmunology: A Current Update of the Interplay between Bone and the Immune System. Front. Immunol. 2020, 11, 58. [Google Scholar] [CrossRef]
- Brylka, L.J.; Schinke, T. Chemokines in Physiological and Pathological Bone Remodeling. Front. Immunol. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Kwee, B.J.; Mooney, D.J.; Duda, G.N. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue Eng. Part B Rev. 2015, 21, 354–364. [Google Scholar] [CrossRef]
- Motoki, D.S.; Mulliken, J.B. The healing of bone and cartilage. Clin. Plast. Surg. 1990, 17, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2, A Review of Their Potential for Clinical Development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. RANKL as the master regulator of osteoclast differentiation. J. Bone Miner. Metab. 2021, 39, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Coury, F.; Peyruchaud, O.; Machuca-Gayet, I. Osteoimmunology of Bone Loss in Inflammatory Rheumatic Diseases. Front. Immunol. 2019, 10, 679. [Google Scholar] [CrossRef]
- Tsukasaki, M. RANKL and osteoimmunology in periodontitis. J. Bone Miner. Metab. 2021, 39, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, L.; Sun, Y.; Wang, W.; Wang, X.; Ye, Y.; Chen, X.; Xu, Y. IL-17 regulates the expressions of RANKL and OPG in human periodontal ligament cells via TRAF6/TBK1-JNK/NF-κB pathways. Immunology 2014, 144, 472–485. [Google Scholar] [CrossRef]
- Alvarez, C.; Monasterio, G.; Cavalla, F.; Córdova, L.A.; Hernández, M.; Heymann, D.; Garlet, G.P.; Sorsa, T.; Pärnänen, P.; Lee, H.M.; et al. Osteoimmunology of oral and maxillofacial diseases: Translational applications based on biological mechanisms. Front. Immunol. 2019, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Amengual-Peñafiel, L.; Córdova, L.A.; Jara-Sepúlveda, M.C.; Brañes-Aroca, M.; Marchesani-Carrasco, F.; Cartes-Velásquez, R. Osteoimmunology drives dental implant osseointegration: A new paradigm for implant dentistry. Jpn. Dent. Sci. Rev. 2021, 57, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Olmedo, D.G. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol. 2000 2001, 86, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Ren, F.; Ye, Y.; Wang, F.; Zheng, C.; Qian, Y.; Zhang, M. The Macrophage-Osteoclast Axis in Osteoimmunity and Osteo-Related Diseases. Front. Immunol. 2021, 12, 664871. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.A.; de Oliveira Alves, R.; Nascimento, I.M.; Hidalgo, M.A.R.; Scarel-Caminaga, R.M.; Cristina Pigossi, S. Pro- and anti-inflammatory cytokines and osteoclastogenesis-related factors in peri-implant diseases: Systematic review and meta-analysis. BMC Oral Health 2023, 23, 420. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Shim, J.-H. Osteoimmunology: A Brief Introduction. Immune Netw. 2013, 13, 111–115. [Google Scholar] [CrossRef]
- Albrektsson, T.; Tengvall, P.; Amengual, L.; Coli, P.; Kotsakis, G.A.; Cochran, D. Osteoimmune regulation underlies oral implant osseointegration and its perturbation. Front. Immunol. 2023, 13, 1056914. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. ROS. Curr. Biol. 2013, 23, R100–R102. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Migliaccio, E.; Orsini, F.; Paolucci, D.; Moroni, M.; Contursi, C.; Pelliccia, G.; Luzi, L.; Minucci, S.; Marcaccio, M.; et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005, 122, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Pula, G. The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef] [PubMed]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Sardaro, N.; Della Vella, F.; Incalza, M.A.; DIStasio, D.; Lucchese, A.; Contaldo, M.; Laudadio, C.; Petruzzi, M. Oxidative Stress and Oral Mucosal Diseases: An Overview. In Vivo 2019, 33, 289–296. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- De Boer, J.; Andressoo, J.O.; de Wit, J.; Huijmans, J.; Beems, R.B.; van Steeg, H.; Weeda, G.; van der Horst, G.T.; van Leeuwen, W.; Themmen, A.P.; et al. Premature aging in mice deficient in DNA repair and transcription. Science 2002, 296, 1276–1279. [Google Scholar] [CrossRef]
- Iantomasi, T.; Favilli, F.; Catarzi, S.; Vincenzini, M.T. GSH role on platelet-derived growth factor receptor tyrosine phosphorylation induced by H2O2. Biochem. Biophys. Res. Commun. 2001, 280, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- A Blanco, R.; Ziegler, T.R.; A Carlson, B.; Cheng, P.-Y.; Park, Y.; A Cotsonis, G.; Accardi, C.J.; Jones, D.P. Diurnal variation in glutathione and cysteine redox states in human plasma. Am. J. Clin. Nutr. 2007, 86, 1016–1023. [Google Scholar] [CrossRef]
- Koh, J.M.; Lee, Y.S.; Byun, C.H.; Chang, E.J.; Kim, H.; Kim, Y.H.; Kim, H.H.; Kim, G.S. Alpha-lipoic acid suppresses osteoclastogenesis despite increasing the receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio in human bone marrow stromal cells. J. Endocrinol. 2005, 185, 401–413. [Google Scholar] [CrossRef]
- Rinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar]
- Fontani, F.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Glutathione, N-acetylcysteine and lipoic acid down-regulate starvation-induced apoptosis, RANKL/OPG ratio and sclerostin in osteocytes: Involvement of JNK and ERK1/2 signalling. Calcif. Tissue Int. 2015, 96, 335–346. [Google Scholar] [CrossRef]
- Endur, O.F.; Turan, Y.; Tastaban, E.; Serter, M. Antioxidant status in patients with osteoporosis: A controlled study. Jt. Bone Spine 2009, 76, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Lean, J.M.; Jagger, C.J.; Kirstein, B.; Fuller, K.; Chambers, T.J. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 2005, 146, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Naka, K.; Muraguchi, T.; Hoshii, T.; Hirao, A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008, 10, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Matteo, M.; Rollo, T.; De Rosario, F.; Greco, P.; Vendemiale, G.; Serviddio, G. Sex hormones modulate circulating antioxidant enzymes: Impact of estrogen therapy. Redox Biol. 2013, 1, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Savasky, B.M.; Mascotti, D.P.; Patel, N.; Rodriguez-Collazo, E. Nutritional and Pharmacological Effects on Oxidative Stress in Soft Tissue and Bone Remodeling. J. Nutr. Metab. 2018, 2018, 4183407. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C. Bone: Oxidative stress and osteoporosis. Nat. Rev. Endocrinol. 2014, 10, 3. [Google Scholar] [PubMed]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investg. 2003, 112, 915–923. [Google Scholar] [CrossRef]
- Filaire, E.; Toumi, H. Reactive oxygen species and exercise on bone metabolism: Friend or enemy? Jt. Bone Spine 2012, 79, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Martin-Millan, M.; Ambrogini, E.; Bradsher, R., 3rd; Han, L.; Chen, X.D.; Roberson, P.K.; Weinstein, R.S.; O’Brien, C.A.; Jilka, R.L.; et al. Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERalpha. J. Bone Miner. Res. 2010, 25, 769–781. [Google Scholar] [CrossRef]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef]
- Almeida, M.; Ambrogini, E.; Han, L.; Manolagas, S.C.; Jilka, R.L. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J. Biol. Chem. 2009, 284, 27438–27448. [Google Scholar] [CrossRef]
- Yousefzadeh, G.; Larijani, B.; Mohammadirad, A.; Heshmat, R.; Dehghan, G.; Rahimi, R.; Abdollahi, M. Determination of oxidative stress status and concentration of TGF-beta 1 in the blood and saliva of osteoporotic subjects. Ann. New York Acad. Sci. J. 2006, 1091, 142–150. [Google Scholar] [CrossRef]
- Yang, R.; Li, J.; Zhang, J.; Xue, Q.; Qin, R.; Wang, R.; Goltzman, D.; Miao, D. 17β-estradiol plays the anti-osteoporosis role via a novel ESR1-Keap1-Nrf2 axis-mediated stress response activation and Tmem119 upregulation. Free. Radic. Biol. Med. 2023, 195, 231–244. [Google Scholar] [CrossRef]
- Huang, L.; Lu, S.; Bian, M.; Wang, J.; Yu, J.; Ge, J.; Zhang, J.; Xu, Q. Punicalagin attenuates TNF-α-induced oxidative damage and promotes osteogenic differentiation of bone mesenchymal stem cells by activating the Nrf2/HO-1 pathway. Exp. Cell. Res. 2023, 430, 113717. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Y.; Zhou, X.; Tian, Y.; Miao, Z.; Ko, C.-C.; Hu, X. Nrf2 differentially regulates osteoclast and osteoblast differentiation for bone homeostasis. Biochem. Biophys. Res. Commun. 2023, 674, 19–26. [Google Scholar] [CrossRef]
- Nabeshima, T.; Tsukamoto, M.; Wang, K.-Y.; Mano, Y.; Arakawa, D.; Kosugi, K.; Tajima, T.; Yamanaka, Y.; Suzuki, H.; Kawasaki, M.; et al. Delayed cortical bone healing due to impaired nuclear Nrf2 translocation in COPD mice. Bone 2023, 173, 116804. [Google Scholar] [CrossRef]
- Golbidi, S.; Li, H.; Laher, I. Oxidative Stress: A Unifying Mechanism for Cell Damage Induced by Noise, (Water-Pipe) Smoking, and Emotional Stress-Therapeutic Strategies Targeting Redox Imbalance. Antioxid. Redox Signal. 2018, 28, 741–759. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. Type 2 diabetes as a redox disease. Lancet 2014, 383, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.E.; Coleman, C.M. Impact of Diabetes Mellitus on Bone Health. Int. J. Mol. Sci. 2019, 20, 4873. [Google Scholar] [CrossRef] [PubMed]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Ushiyama, A.; Inaba, Y.; Hattori, K. Increased oxidative stress and effects on inflammatory cytokine secretion by heated tobacco products aerosol exposure to mice. Biochem. Biophys. Res. Commun. 2022, 610, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Rahman, I.; Romanos, G.E. Tobacco-product usage as a risk factor for dental implants. Periodontol. 2000 2019, 81, 48–56. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Vitamin, D. metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, R.; Qiao, W.; Zhang, W.; Chen, J.; Mao, L.; Goltzman, D.; Miao, D. 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell 2019, 18, e12951. [Google Scholar] [CrossRef] [PubMed]
- Ghahramanipour, Z.; Alipour, S.; Masoumi, J.; Rostamlou, A.; Hatami-Sadr, A.; Heris, J.A.; Naseri, B.; Jafarlou, M.; Baradaran, B. Regulation of Dendritic Cell Functions by Vitamins as Promising Therapeutic Strategy for Immune System Disorders. Adv. Biol. 2023, 7, e2300142. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K.; Falch, B.M.; Danielsen, K.; Johannessen, M.; Sollid, J.U.E.; Thune, I.; Grimnes, G.; Jorde, R.; Simonsen, G.S.; Furberg, A.-S. Staphylococcus aureus nasal carriage is associated with serum 25-hydroxyvitamin D levels, gender and smoking status. The Tromsø Staph and Skin Study. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Chen, D.; Zuo, C.; Qin, J.; Wang, H.; Wang, J.; Yu, Y. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J. Biol. Chem. 2014, 289, 11681–11694. [Google Scholar] [CrossRef]
- Flynn, L.; Zimmerman, L.H.; McNorton, K.; Dolman, M.; Tyburski, J.; Baylor, A.; Wilson, R.; Dolman, H. Effects of vitamin D deficiency in critically ill surgical patients. Am. J. Surg. 2012, 203, 379–382, discussion 382. [Google Scholar] [CrossRef]
- Zhou, A.; Hyppönen, E. Vitamin D deficiency and C-reactive protein: A bidirectional Mendelian randomization study. Int. J. Epidemiol. 2023, 52, 260–271. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—A review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Werny, J.G.; Sagheb, K.; Diaz, L.; Kämmerer, P.W.; Al-Nawas, B.; Schiegnitz, E. Does vitamin D have an effect on osseointegration of dental implants? A systematic review. Int. J. Implant Dent. 2022, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Bazal-Bonelli, S.; Sánchez-Labrador, L.; Cortés-Bretón Brinkmann, J.; Cobo-Vázquez, C.; Martínez-Rodríguez, N.; Beca-Campoy, T.; Santos-Marino, J.; Rodríguez-Fernández, E.; Alvarado-Lorenzo, M. Influence of Serum Vitamin D Levels on Survival Rate and Marginal Bone Loss in Dental Implants: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10120. [Google Scholar] [CrossRef]
- Guido Mangano, F.; Ghertasi Oskouei, S.; Paz, A.; Mangano, N.; Mangano, C. Low serum vitamin D and early dental implant failure: Is there a connection? A retrospective clinical study on 1740 implants placed in 885 patients. J. Dent. Res. Dent. Clin. Dent. Prospect. 2018, 12, 174–182. [Google Scholar] [CrossRef]
- Choukroun, J.; Khoury, G.; Khoury, F.; Russe, P.; Testori, T.; Komiyama, Y.; Sammartino, G.; Palacci, P.; Tunali, M.; Choukroun, E. Two neglected biologic risk factors in bone grafting and implantology: High low-density lipoprotein cholesterol and low serum vitamin D. J. Oral Implantol. 2014, 40, 110–114. [Google Scholar] [CrossRef]
- Mandal, C.C. High Cholesterol Deteriorates Bone Health: New Insights into Molecular Mechanisms. Front. Endocrinol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Keuroghlian, A.; Barroso, A.D.V.; Kirikian, G.; Bezouglaia, O.; Tintut, Y.; Tetradis, S.; Moy, P.; Pirih, F.; Aghaloo, T. The effects of hyperlipidemia on implant osseointegration in the mouse femur. J. Oral Implantol. 2015, 41, e7–e11. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.Q.; Brodeur, M.R.; Falstrault, L.; Rhainds, D.; Brissette, L. Expression of caveolin-1 in hepatic cells increases oxidized LDL uptake and preserves the expression of lipoprotein receptors. J. Cell. Biochem. 2009, 108, 906–915. [Google Scholar] [CrossRef]
- French, D.; Noroozi, M.; Shariati, B.; Larjava, H. Clinical retrospective study of self-reported penicillin allergy on dental implant failures and infections. Quintessence Int. 2016, 47, 861–870. [Google Scholar]
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic allergy. Lancet 2019, 393, 183–198. [Google Scholar] [CrossRef]
- Basma, H.S.; Misch, C.M. Extraction Socket Grafting and Ridge Augmentation Failures Associated with Clindamycin Antibiotic Therapy: A Retrospective Study. Int. J. Oral Maxillofac. Implants. 2021, 36, 122–125. [Google Scholar] [CrossRef]
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.S.; Pichler, W.; et al. International Consensus on drug allergy. Allergy 2014, 69, 420–437. [Google Scholar] [CrossRef]
- Bozzetto, S.; Carraro, S.; Giordano, G.; Boner, A.; Baraldi, E. Asthma, allergy and respiratory infections: The vitamin D hypothesis. Allergy 2012, 67, 10–17. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Suppa, M.; Ginaldi, L.; De Martinis, M. Does Allergy Break Bones? Osteoporosis and Its Connection to Allergy. Int. J. Mol. Sci. 2020, 21, 712. [Google Scholar] [CrossRef]
- Habibzay, M.; Saldana, J.I.; Goulding, J.; Lloyd, C.M.; Hussell, T. Altered regulation of Toll-like receptor responses impairs antibacterial immunity in the allergic lung. Mucosal. Immunol. 2012, 5, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Yoshimoto, T.; Kajiya, M.; Ouhara, K.; Matsuda, S.; Takemura, T.; Akutagawa, K.; Takeda, K.; Mizuno, N.; Kurihara, H. Regulation of defensive function on gingival epithelial cells can prevent periodontal disease. Jpn. Dent. Sci. Rev. 2018, 54, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, R.G.; Rana, A.; Sarkar, A. Gingival Biotype Assessment in a Healthy Periodontium: Transgingival Probing Method. J. Clin. Diagn. Res. 2015, 9, ZC66–ZC69. [Google Scholar] [PubMed]
- Tribble, G.D.; Lamont, R.J. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol. 2000 2010, 52, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S15), 32–49. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, A.; Connor, K.M.; Mammoto, T.; Yung, C.W.; Huh, D.; Aderman, C.M.; Mostoslavsky, G.; Smith, L.E.; Ingber, D.E. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 2009, 457, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, X.; Yang, C.; Su, P.; Yin, C.; Qian, A.R. The Impact of Oxidative Stress on the Bone System in Response to the Space Special Environment. Int. J. Mol. Sci. 2017, 18, 2132. [Google Scholar] [CrossRef]
- Duyck, J.; Roesems, R.; Cardoso, M.V.; Ogawa, T.; De Villa Camargos, G.; Vandamme, K. Effect of insertion torque on titanium implant osseointegration: An animal experimental study. Clin. Oral Implant. Res. 2015, 26, 191–196. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Roccuzzo, A.; Marruganti, C.; Fickl, S. The importance of soft tissue condition in bone regenerative procedures to ensure long-term peri-implant health. Periodontol. 2000 2023, 93, 129–138. [Google Scholar] [CrossRef]
- Plonka, A.B.; Sheridan, R.A.; Wang, H.L. Flap Designs for Flap Advancement During Implant Therapy: A Systematic Review. Implant Dent. 2017, 26, 145–152. [Google Scholar] [CrossRef]
- Brito, C.; Tenenbaum, H.C.; Wong, B.K.; Schmitt, C.; Nogueira-Filho, G. Is keratinized mucosa indispensable to maintain peri-implant health? A systematic review of the literature. J. Biomed Mater. Res. B Appl. Biomater. 2014, 102, 643–650. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Schwarz, F.; Sader, R. Influence of width of keratinized tissue on the prevalence of peri-implant diseases: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2022, 33 (Suppl. S23), 8–31. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Fabrizi, G.; Bruno, C. Protective effects of oral antioxidants on skin and eye function. Skinmed 2004, 3, 310–316. [Google Scholar]
- McCarty, M.F.; Lewis Lujan, L.; Iloki Assanga, S. Targeting Sirt1, AMPK, Nrf2, CK2, and Soluble Guanylate Cyclase with Nutraceuticals: A Practical Strategy for Preserving Bone Mass. Int. J. Mol. Sci. 2022, 23, 4776. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid. Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Pinnell, S.R. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J. Biol. Med. 1985, 58, 553–559. [Google Scholar]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Barrios-Garay, K.; Toledano-Serrabona, J.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Clinical effect of vitamin C supplementation on bone healing: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2022, 27, e205–e215. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, G.J.; Yoo, H.S.; Song, D.H.; Chung, K.H.; Lee, K.J.; Koo, Y.T.; An, J.H. Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways. Nutrients 2019, 11, 506. [Google Scholar] [CrossRef]
- Li, X.; Tang, L.; Lin, Y.F.; Xie, G.F. Role of vitamin C in wound healing after dental implant surgery in patients treated with bone grafts and patients with chronic periodontitis. Clin. Implant Dent. Relat. Res. 2018, 20, 793–798. [Google Scholar] [CrossRef]
- Tada, A.; Miura, H. The Relationship between Vitamin C and Periodontal Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2472. [Google Scholar] [CrossRef] [PubMed]
- Ustianowski, Ł.; Ustianowska, K.; Gurazda, K.; Rusiński, M.; Ostrowski, P.; Pawlik, A. The Role of Vitamin C and Vitamin D in the Pathogenesis and Therapy of Periodontitis-Narrative Review. Int. J. Mol. Sci. 2023, 24, 6774. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Beveridge, S.; Suter, M. A combination of high-dose vitamin C plus zinc for the common cold. J. Int. Med. Res. 2012, 40, 28–42. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Balmik, A.A.; Chinnathambi, S. Melatonin Reduces GSK3β-Mediated Tau Phosphorylation, Enhances Nrf2 Nuclear Translocation and Anti-Inflammation. ASN Neuro. 2020, 12, 1759091420981204. [Google Scholar] [CrossRef]
- Zheng, S.; Zhou, C.; Yang, H.; Li, J.; Feng, Z.; Liao, L.; Li, Y. Melatonin Accelerates Osteoporotic Bone Defect Repair by Promoting Osteogenesis-Angiogenesis Coupling. Front. Endocrinol. 2022, 13, 826660. [Google Scholar] [CrossRef]
- Wu, X.; Qiao, S.; Wang, W.; Zhang, Y.; Shi, J.; Zhang, X.; Gu, W.; Zhang, X.; Li, Y.; Ding, X.; et al. Melatonin prevents peri-implantitis via suppression of TLR4/NF-κB. Acta Biomater. 2021, 134, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Permuy, M.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Melatonin: A Review of Its Potential Functions and Effects on Dental Diseases. Int. J. Mol. Sci. 2017, 18, 865. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moreno, G.; Aguilar-Salvatierra, A.; Boquete-Castro, A.; Guardia, J.; Piattelli, A.; Perrotti, V.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L. Outcomes of topical applications of melatonin in implant dentistry: A systematic review. Implant Dent. 2015, 24, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kiran, S.; Bammidi, N.; Kumar, A.K.; Kumar, P.S.; Karnam, Y. Evaluation of the Effect of Topical Melatonin Application on Immediately Placed Dental Implants Using Cone Beam Computed Tomography (CBCT). Cureus 2022, 14, e25233. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Nie, P.; Lou, Y.; Bai, X.; Zhu, Y.; Guo, Q.; Luo, P.; Zhang, W.; Li, B. Influence of zinc levels and Nrf2 expression in the clinical and pathological changes in patients with diabetic nephropathy. Nutr. Diabetes 2022, 12, 37. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Jin, Y.; Liu, T.; Wang, W.; Lu, X.; Zhang, C. Zinc Supplementation Prevented Type 2 Diabetes-Induced Liver Injury Mediated by the Nrf2-MT Antioxidative Pathway. J. Diabetes Res. 2021, 2021, 6662418. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Romeo, J.; Malavolta, M.; Costarelli, L.; Giacconi, R.; Diaz, L.E.; Marcos, A. Zinc: Dietary intake and impact of supplementation on immune function in elderly. Age 2013, 35, 839–860. [Google Scholar] [CrossRef]
- Hsu, E.; Pacifici, R. From Osteoimmunology to Osteomicrobiology: How the Microbiota and the Immune System Regulate Bone. Calcif. Tissue Int. 2018, 102, 512–521. [Google Scholar] [CrossRef]
- Ohlsson, C.; Sjögren, K. Effects of the gut microbiota on bone mass. Trends Endocrinol. Metab. 2015, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, X.; Liu, C.; Xu, X. Oral Osteomicrobiology: The Role of Oral Microbiota in Alveolar Bone Homeostasis. Front. Cell. Infect. Microbiol. 2021, 11, 751503. [Google Scholar] [CrossRef] [PubMed]
- Karaca, B.; Yilmaz, M.; Gursoy, U.K. Targeting Nrf2 with Probiotics and Postbiotics in the Treatment of Periodontitis. Biomolecules 2022, 12, 729. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P.A. Novel role of vitamin K in preventing oxidative injury to developing oligodendrocytes and neurons. J. Neurosci. 2003, 23, 5816–5826. [Google Scholar] [CrossRef]
- Akbari, S.; Rasouli-Ghahroudi, A.A. Vitamin K and Bone Metabolism: A Review of the Latest Evidence in Preclinical Studies. Biomed Res. Int. 2018, 2018, 4629383. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Vallibhakara, S.A.; Nakpalat, K.; Sophonsritsuk, A.; Tantitham, C.; Vallibhakara, O. Effect of Vitamin E Supplement on Bone Turnover Markers in Postmenopausal Osteopenic Women: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients 2021, 13, 4226. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A.; Peroni, G. Copper as Dietary Supplement for Bone Metabolism: A Review. Nutrients 2021, 13, 2246. [Google Scholar] [CrossRef]
- Ciosek, Ż.; Kot, K.; Rotter, I. Iron, Zinc, Copper, Cadmium, Mercury, and Bone Tissue. Int. J. Environ. Res. Public Health 2023, 20, 2197. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Zhang, K.; Wei, Y.; Hua, W.; Gao, Y.; Li, X.; Ye, L. Therapeutic potentials and modulatory mechanisms of fatty acids in bone. Cell. Prolif. 2020, 53, e12735. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Sahni, S.; Kerstetter, J.E.; Kenny, A.M.; Hannan, M.T. Polyunsaturated fatty acids and their relation with bone and muscle health in adults. Curr. Osteoporos. Rep. 2013, 11, 203–212. [Google Scholar] [CrossRef]
- Nastri, L.; Moretti, A.; Migliaccio, S.; Paoletta, M.; Annunziata, M.; Liguori, S.; Toro, G.; Bianco, M.; Cecoro, G.; Guida, L.; et al. Do Dietary Supplements and Nutraceuticals Have Effects on Dental Implant Osseointegration? A Scoping Review. Nutrients 2020, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Duve, C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J. Cell. Biol. 1967, 33, 437–449. [Google Scholar]
- Ohsumi, Y.; Mizushima, N. Two ubiquitin-like conjugation systems essential for autophagy. Semin. Cell. Dev. Biol. 2004, 15, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xiao, Y. The Autophagy in Osteoimmonology: Self-Eating, Maintenance, and Beyond. Front. Endocrinol. 2019, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.J.; Rao, S.S.; Tan, Y.J.; Yin, H.; Hu, X.K.; Zhang, Y.; Liu, Y.W.; Yue, T.; Chen, L.J.; Li, L.; et al. Fasting before or after wound injury accelerates wound healing through the activation of pro-angiogenic SMOC1 and SCG2. Theranostics 2020, 10, 3779–3792. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, S.; Uetake, T.; Hagiwara, K.; Hirayama, M.; Hus, L.H.; Kimura, H.; Sugiyama, Y. Clinical effects of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis. Nihon Kyobu Shikkan Gakkai Zasshi 1987, 25, 632–642. [Google Scholar] [PubMed]
- McDonald, P.J.; Pruul, H. Phagocyte uptake and transport of azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Kuo, M.L.; Hsiao, H.S.; Lee, P.T. Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4+ T cells. Int. Immunopharmacol. 2016, 40, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Patel, A. Azithromycin in Combination with Ceftriaxone Reduces Systemic Inflammation and Provides Survival Benefit in a Murine Model of Polymicrobial Sepsis. Antimicrob. Agents. Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Andrada, A.C.; Azuma, M.M.; Furusho, H.; Hirai, K.; Xu, S.; White, R.R.; Sasaki, H. Immunomodulation Mediated by Azithromycin in Experimental Periapical Inflammation. J. Endod. 2020, 46, 1648–1654. [Google Scholar] [CrossRef]
- Xing, Y.W.; Liu, K.Z. Azithromycin inhibited oxidative stress and apoptosis of high glucose-induced podocytes by inhibiting STAT1 pathway. Drug Dev. Res. 2021, 82, 990–998. [Google Scholar] [CrossRef]
- Song, Y. Azithromycin ameliorated cigarette smoke-induced airway epithelial barrier dysfunction by activating Nrf2/GCL/GSH signaling pathway. Respir. Res. 2023, 24, 69. [Google Scholar] [CrossRef]
- Fernandez, A. Azithromycin modulates murine immune responses to pneumococcal conjugate vaccine and inhibits nasal clearance of bacteria. J. Infect. Dis. 2004, 190, 1762–1766. [Google Scholar] [CrossRef]
- Nagano, T.; Yamaguchi, T.; Kajiyama, S.; Suzuki, T.; Matsushima, Y.; Yashima, A.; Shirakawa, S.; Gomi, K. Effect of Azithromycin on Proinflammatory Cytokine Production in Gingival Fibroblasts and the Remodeling of Periodontal Tissue. J. Clin. Med. 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.; Walters, J.D. Inhibition of neutrophil inflammatory mediator expression by azithromycin. Clin. Oral. Investig. 2020, 24, 4493–4500. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Adda, F.; Shoeffler, C.; Vervelle, A. PRF Platelet Rich Fibrin: An opportunity in implantology. Implantodontie 2001, 41, 55–62. [Google Scholar]
- Herrera-Vizcaíno, C.; Dohle, E.; Al-Maawi, S.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Choukroun, J.; Ghanaati, S. Platelet-rich fibrin secretome induces three-dimensional angiogenic activation in vitro. Eur. Cell. Mater. 2019, 37, 250–264. [Google Scholar] [CrossRef]
- Nasirzade, J.; Kargarpour, Z.; Hasannia, S.; Strauss, F.J.; Gruber, R. Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro. J. Periodontol. 2020, 91, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, F.; Edwards, J.; Zou, L.; Kaufman, D.S. Fibrinogen Induces RUNX2 Activity and Osteogenic Development from Human Pluripotent Stem Cells. Stem Cells 2016, 34, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Kargarpour, Z.; Nasirzade, J.; Strauss, F.J.; Di Summa, F.; Hasannia, S.; Müller, H.D.; Gruber, R. Platelet-rich fibrin suppresses in vitro osteoclastogenesis. J. Periodontol. 2020, 91, 413–421. [Google Scholar] [CrossRef]
- Kargarpour, Z.; Nasirzade, J.; Panahipour, L.; Miron, R.J.; Gruber, R. Platelet-Rich Fibrin Decreases the Inflammatory Response of Mesenchymal Cells. Int. J. Mol. Sci. 2021, 22, 11333. [Google Scholar] [CrossRef]
- Kargarpour, Z.; Panahipour, L.; Mildner, M.; Miron, R.J.; Gruber, R. Lipids of Platelet-Rich Fibrin Reduce the Inflammatory Response in Mesenchymal Cells and Macrophages. Cells 2023, 12, 634. [Google Scholar] [CrossRef]
- Sordi, M.B.; Panahipour, L.; Kargarpour, Z.; Gruber, R. Platelet-Rich Fibrin Reduces IL-1β Release from Macrophages Undergoing Pyroptosis. Int. J. Mol. Sci. 2022, 23, 8306. [Google Scholar] [CrossRef]
- Kargarpour, Z.; Nasirzade, J.; Di Summa, F.; Panahipour, L.; Miron, R.J.; Gruber, R. Platelet-Rich Fibrin Can Neutralize Hydrogen Peroxide-Induced Cell Death in Gingival Fibroblasts. Antioxidants 2020, 9, 560. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Jimbo, R.; Barbé, G.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF). Curr. Pharm. Biotechnol. 2012, 13, 1145–1152. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin with the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Cascalho, M.; Platt, J.L. The immunological barrier to xenotransplantation. Immunity 2001, 14, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yang, S.; Ni, G.; Ji, J.; Luo, M.; Du, W. The Preparation and Effects of Organic-Inorganic Antioxidative Biomaterials for Bone Repair. Biomedicines 2023, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, P.; Ren, H.; Ding, Z.; Yan, Y.; Li, S.; Yin, J. Effects of bovine cancellous bone powder/poly amino acid composites on cellular behaviors and osteogenic performances. Biomed Mater. 2021, 16, 055002. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Wong, J.L.; Vapniarsky, N.; Griffiths, L.G. In vivo xenogeneic scaffold fate is determined by residual antigenicity and extracellular matrix preservation. Biomaterials 2016, 92, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taveedach, B.; Suwanwela, J. Inflammation-Related Gene Profile Histology and Immunohistochemistry of Soft Connective Tissue Covering Bone Grafted with Deproteinized Bovine Bone Mineral and Demineralized Freeze-Dried Bone Allograft. Int. J. Oral Maxillofac. Implant. 2021, 36, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.G.; Sykes, M. Xenotransplantation: Current status and a perspective on the future. Nat. Rev. Immunol. 2007, 07, 519–531. [Google Scholar] [CrossRef]
- Cohen, O.; Ormianer, Z.; Tal, H.; Rothamel, D.; Weinreb, M.; Moses, O. Differences in crestal bone-to-implant contact following an under-drilling compared to an over-drilling protocol. A study in the rabbit tibia. Clin. Oral. Investig. 2016, 20, 2475–2480. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Walboomers, X.F.; Jansen, J.A. Biological limits of the undersized surgical technique: A study in goats. Clin. Oral Implant. Res. 2011, 22, 129–134. [Google Scholar] [CrossRef]
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Gannon, S.C.; Cantley, M.D.; Haynes, D.R.; Hirsch, R.; Bartold, P.M. Azithromycin suppresses human osteoclast formation and activity in vitro. J. Cell. Physiol. 2013, 228, 1098–1107. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choukroun, E.; Parnot, M.; Surmenian, J.; Gruber, R.; Cohen, N.; Davido, N.; Simonpieri, A.; Savoldelli, C.; Afota, F.; El Mjabber, H.; et al. Bone Formation and Maintenance in Oral Surgery: The Decisive Role of the Immune System—A Narrative Review of Mechanisms and Solutions. Bioengineering 2024, 11, 191. https://doi.org/10.3390/bioengineering11020191
Choukroun E, Parnot M, Surmenian J, Gruber R, Cohen N, Davido N, Simonpieri A, Savoldelli C, Afota F, El Mjabber H, et al. Bone Formation and Maintenance in Oral Surgery: The Decisive Role of the Immune System—A Narrative Review of Mechanisms and Solutions. Bioengineering. 2024; 11(2):191. https://doi.org/10.3390/bioengineering11020191
Chicago/Turabian StyleChoukroun, Elisa, Maximilien Parnot, Jerome Surmenian, Reinhard Gruber, Nicolas Cohen, Nicolas Davido, Alain Simonpieri, Charles Savoldelli, Franck Afota, Hicham El Mjabber, and et al. 2024. "Bone Formation and Maintenance in Oral Surgery: The Decisive Role of the Immune System—A Narrative Review of Mechanisms and Solutions" Bioengineering 11, no. 2: 191. https://doi.org/10.3390/bioengineering11020191