Comparison of Mid-Infrared Handheld and Benchtop Spectrometers to Detect Staphylococcus epidermidis in Bone Grafts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Development of Biofilm on Bone Allografts
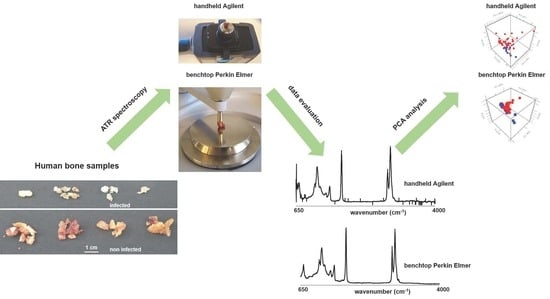
2.3. Benchtop Perkin Elmer Spectrum 100 ATR-IR Spectrometer
2.4. Agilent 4300 Handheld FTIR
2.5. Data Processing
2.6. Principal Component Analyses (PCA)
3. Results
3.1. Spectroscopy Data Evaluation
3.2. Diagnostic Performance PCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts: What they can offer and what they cannot. J. Bone. Joint Surg. Br. 2007, 89, 574–579. [Google Scholar] [CrossRef]
- Zwitser, E.W.; Jiya, T.U.; George Licher, H.; van Royen, B.J. Design and management of an orthopaedic bone bank in The Netherlands. Cell Tissue Bank 2012, 13, 63–69. [Google Scholar] [CrossRef]
- Nodzo, S.R.; Boyle, K.K.; Pavlesen, S.; Rachala, S. Bone morphogenic protein-2 use in revision total hip arthroplasty with acetabular defects. Int. Orthop. 2018, 42, 783–789. [Google Scholar] [CrossRef]
- Coraca-Huber, D.C.; Hausdorfer, J.; Fille, M.; Nogler, M. Effect of storage temperature on gentamicin release from antibiotic-coated bone chips. Cell Tissue Bank 2013, 14, 395–400. [Google Scholar] [CrossRef]
- Hinsenkamp, M.; Muylle, L.; Eastlund, T.; Fehily, D.; Noel, L.; Strong, D.M. Adverse reactions and events related to musculoskeletal allografts: Reviewed by the World Health Organisation Project NOTIFY. Int. Orthop. 2012, 36, 633–641. [Google Scholar] [CrossRef]
- Lewis, C.S.; Katz, J.; Baker, M.I.; Supronowicz, P.R.; Gill, E.; Cobb, R.R. Local antibiotic delivery with bovine cancellous chips. J. Biomater. Appl. 2011, 26, 491–506. [Google Scholar] [CrossRef]
- Sommerfeldt, D.W.; Linhart, W.; Schmandra, T.C.; Konold, P.; Rueger, J.M. Die Knochenbank Richtlinien—Probleme—Anwendung. Unfallchirurgie 1998, 24, 236–244. [Google Scholar] [CrossRef]
- Slooff, T.J.; Buma, P.; Schreurs, B.W.; Schimmel, J.W.; Huiskes, R.; Gardeniers, J. Acetabular and femoral reconstruction with impacted graft and cement. Clin. Orthop. Relat. Res. 1996, 323, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Brewster, N.T.; Gillespie, W.J.; Howie, C.R.; Madabhushi, S.P.; Usmani, A.S.; Fairbairn, D.R. Mechanical considerations in impaction bone grafting. J. Bone. Joint Surg. Br. 1999, 81, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Malkani, A.L.; Voor, M.J.; Fee, K.A.; Bates, C.S. Femoral component revision using impacted morsellised cancellous graft. A biomechanical study of implant stability. J. Bone. Joint Surg. Br. 1996, 78, 973–978. [Google Scholar] [CrossRef]
- Putzer, D.; Mayr, E.; Haid, C.; Reinthaler, A.; Nogler, M. Impaction bone grafting: A laboratory comparison of two methods. J. Bone. Joint Surg. Br. 2011, 93, 1049–1053. [Google Scholar] [CrossRef]
- Putzer, D.; Coraca-Huber, D.; Wurm, A.; Schmoelz, W.; Nogler, M. Optimizing the grain size distribution of allografts in bone impaction grafting. J. Orthop. Res. 2014, 32, 1024–1029. [Google Scholar] [CrossRef]
- Gonzaga, M.G.; Dos Santos Kotake, B.G.; de Figueiredo, F.A.T.; Feldman, S.; Ervolino, E.; Dos Santos, M.C.G.; Issa, J.P.M. Effectiveness of rhBMP-2 association to autogenous, allogeneic, and heterologous bone grafts. Microsc. Res. Tech. 2019, 82, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Coraca-Huber, D.C.; Hausdorfer, J.; Fille, M.; Steidl, M.; Nogler, M. Effect of two cleaning processes for bone allografts on gentamicin impregnation and in vitro antibiotic release. Cell Tissue Bank 2013, 14, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Wurm, A.; Nogler, M.; Ammann, C.G.; Coraca-Huber, D.C. Effect of storage temperature and antibiotic impregnation on the quantity of bone morphogenetic protein seven in human bone grafts. Int. Orthop. 2014, 38, 1513–1517. [Google Scholar] [CrossRef]
- Patel, R. Periprosthetic Joint Infection. N. Engl. J. Med. 2023, 388, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, S.; Wernly, D.; Moerenhout, K.; Trampuz, A.; Borens, O. Infection after fracture fixation. EFORT Open Rev. 2019, 4, 468–475. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e3. [Google Scholar] [CrossRef] [PubMed]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.A.M.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.H.J.; et al. General treatment principles for fracture-related infection: Recommendations from an international expert group. Arch. Orthop. Trauma Surg. 2020, 140, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Grieb, T.A.; Forng, R.Y.; Stafford, R.E.; Lin, J.; Almeida, J.; Bogdansky, S.; Ronholdt, C.; Drohan, W.N.; Burgess, W.H. Effective use of optimized, high-dose (50 kGy) gamma irradiation for pathogen inactivation of human bone allografts. Biomaterials 2005, 26, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- DePaula, C.A.; Truncale, K.G.; Gertzman, A.A.; Sunwoo, M.H.; Dunn, M.G. Effects of hydrogen peroxide cleaning procedures on bone graft osteoinductivity and mechanical properties. Cell Tissue Bank 2005, 6, 287–298. [Google Scholar] [CrossRef]
- Vastel, L.; Meunier, A.; Siney, H.; Sedel, L.; Courpied, J.P. Effect of different sterilization processing methods on the mechanical properties of human cancellous bone allografts. Biomaterials 2004, 25, 2105–2110. [Google Scholar] [CrossRef]
- Yamamoto, T.; Uchida, K.; Naruse, K.; Suto, M.; Urabe, K.; Uchiyama, K.; Suto, K.; Moriya, M.; Itoman, M.; Takaso, M. Quality assessment for processed and sterilized bone using Raman spectroscopy. Cell Tissue Bank 2012, 13, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.M.; McLaren, A.C.; Sculco, T.P.; Brause, B.; Bostrom, M.; Kates, S.L.; Parvizi, J.; Alt, V.; Arnold, W.V.; Carli, A.; et al. Adjuvant antibiotic-loaded bone cement: Concerns with current use and research to make it work. J. Orthop. Res. 2021, 39, 227–239. [Google Scholar] [CrossRef]
- Saeed, K.; McLaren, A.C.; Schwarz, E.M.; Antoci, V.; Arnold, W.V.; Chen, A.F.; Clauss, M.; Esteban, J.; Gant, V.; Hendershot, E.; et al. 2018 international consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. J. Orthop. Res. 2019, 37, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Knabl, L.; Kuppelwieser, B.; Mayr, A.; Posch, W.; Lackner, M.; Coraa-Huber, D.; Danita, A.; Blauth, M.; Lass-Florl, C.; Orth-Holler, D. High percentage of microbial colonization of osteosynthesis material in clinically unremarkable patients. Microbiologyopen 2019, 8, e00658. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; An, Y.H.; Baldassarri, L.; Pirini, V.; Donati, M.E.; Pegreffi, F.; Montanaro, L. Prevalence and antibiotic resistance of 15 minor staphylococcal species colonizing orthopedic implants. Int. J. Artif. Organs. 2006, 29, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; von Eiff, C.; Pirini, V.; Ravaioli, S.; Becker, K.; Arciola, C.R. Cluster analysis of ribotyping profiles of Staphylococcus epidermidis isolates recovered from foreign body-associated orthopedic infections. J. Biomed. Mater. Res. A 2009, 88, 664–672. [Google Scholar] [CrossRef]
- Kresken, M.; Hafner, D. Drug resistance among clinical isolates of frequently encountered bacterial species in central Europe during 1975–1995. Study Group Bacterial Resistance of the Paul-Ehrlich-Society for Chemotherapy. Infection 1999, 27 (Suppl. S2), S2–S8. [Google Scholar] [CrossRef]
- Giormezis, N.; Kolonitsiou, F.; Foka, A.; Drougka, E.; Liakopoulos, A.; Makri, A.; Papanastasiou, A.D.; Vogiatzi, A.; Dimitriou, G.; Marangos, M.; et al. Coagulase-negative staphylococcal bloodstream and prosthetic-device-associated infections: The role of biofilm formation and distribution of adhesin and toxin genes. J. Med. Microbiol. 2014, 63, 1500–1508. [Google Scholar] [CrossRef]
- McCreadie, B.R.; Morris, M.D.; Chen, T.C.; Sudhaker Rao, D.; Finney, W.F.; Widjaja, E.; Goldstein, S.A. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone 2006, 39, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Gamsjaeger, S.; Buchinger, B.; Zoehrer, R.; Phipps, R.; Klaushofer, K.; Paschalis, E.P. Effects of one year daily teriparatide treatment on trabecular bone material properties in postmenopausal osteoporotic women previously treated with alendronate or risedronate. Bone 2011, 49, 1160–1165. [Google Scholar] [CrossRef]
- Esmonde-White, K.A.; Esmonde-White, F.W.; Holmes, C.M.; Morris, M.D.; Roessler, B.J. Alterations to bone mineral composition as an early indication of osteomyelitis in the diabetic foot. Diabetes Care 2013, 36, 3652–3654. [Google Scholar] [CrossRef] [PubMed]
- Gamsjaeger, S.; Hofstetter, B.; Zwettler, E.; Recker, R.; Gasser, J.A.; Eriksen, E.F.; Klaushofer, K.; Paschalis, E.P. Effects of 3 years treatment with once-yearly zoledronic acid on the kinetics of bone matrix maturation in osteoporotic patients. Osteoporos. Int. 2013, 24, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Busse, B.; Bale, H.A.; Zimmermann, E.A.; Panganiban, B.; Barth, H.D.; Carriero, A.; Vettorazzi, E.; Zustin, J.; Hahn, M.; Ager, J.W., 3rd; et al. Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci. Transl. Med. 2013, 5, 193ra188. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, C.; Falgayrac, G.; During, A.; Vieillard, M.H.; Maes, J.M.; Cortet, B.; Penel, G. Molecular alterations of bone quality in sequesters of bisphosphonates-related osteonecrosis of the jaws. Osteoporos. Int. 2014, 25, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Imbert, L.; Auregan, J.C.; Pernelle, K.; Hoc, T. Mechanical and mineral properties of osteogenesis imperfecta human bones at the tissue level. Bone 2014, 65, 18–24. [Google Scholar] [CrossRef]
- Khalid, M.; Bora, T.; Ghaithi, A.A.; Thukral, S.; Dutta, J. Raman Spectroscopy detects changes in Bone Mineral Quality and Collagen Cross-linkage in Staphylococcus Infected Human Bone. Sci. Rep. 2018, 8, 9417. [Google Scholar] [CrossRef] [PubMed]
- Wurm, A.; Kuhn, J.; Kugel, K.; Putzer, D.; Arora, R.; Coraca-Huber, D.C.; Zelger, P.; Badzoka, J.; Kappacher, C.; Huck, C.W.; et al. Raman microscopic spectroscopy as a diagnostic tool to detect Staphylococcus epidermidis in bone grafts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121570. [Google Scholar] [CrossRef]
- Wurm, A.; Steiger, R.; Ammann, C.G.; Putzer, D.; Liebensteiner, M.C.; Nogler, M.; Coraca-Huber, D.C. Changes in the Chemical Quality of Bone Grafts During Clinical Preparation Detected by Raman Spectroscopy. Biopreservation Biobanking 2016, 14, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Lasch, P.; Beekes, M.; Schmitt, J.; Naumann, D. Biomedical Vibrational Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Salzer, R.; Siesler, H.W. Infrared and Raman Spectroscopic Imaging; Vch Pub: Weinheim, Germany, 2009. [Google Scholar]
- Hutengs, C.; Ludwig, B.; Jung, A.; Eisele, A.; Vohland, M. Comparison of Portable and Bench-Top Spectrometers for Mid-Infrared Diffuse Reflectance Measurements of Soils. Sensors 2018, 18, 993. [Google Scholar] [CrossRef] [PubMed]
- Bec, K.B.; Grabska, J.; Huck, C.W. Principles and Applications of Miniaturized Near-Infrared (NIR) Spectrometers. Chemistry 2021, 27, 1514–1532. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, M.; Krafft, C.; Ditzel, H.J.; Johansen, L.E.; Hassing, S.; Popp, J. Discriminating isogenic cancer cells and identifying altered unsaturated fatty acid content as associated with metastasis status, using k-means clustering and partial least squares-discriminant analysis of Raman maps. Anal. Chem. 2010, 82, 2797–2802. [Google Scholar] [CrossRef]
- Swain, R.J.; Stevens, M.M. Raman microspectroscopy for non-invasive biochemical analysis of single cells. Biochem. Soc. Trans. 2007, 35, 544–549. [Google Scholar] [CrossRef]
- Morris, M.D.; Mandair, G.S. Raman assessment of bone quality. Clin. Orthop. Relat. Res. 2011, 469, 2160–2169. [Google Scholar] [CrossRef]
- Gasior-Glogowska, M.; Komorowska, M.; Hanuza, J.; Ptak, M.; Kobielarz, M. Structural alteration of collagen fibres--spectroscopic and mechanical studies. Acta Bioeng. Biomech. 2010, 12, 55–62. [Google Scholar]
- Maiti, N.C.; Apetri, M.M.; Zagorski, M.G.; Carey, P.R.; Anderson, V.E. Raman spectroscopic characterization of secondary structure in natively unfolded proteins: Alpha-synuclein. J. Am. Chem. Soc. 2004, 126, 2399–2408. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Happillon, T.; Feru, J.; Brassart-Passco, S.; Angiboust, J.-F.; Manfait, M.; Piot, O. Raman comparison of skin dermis of different ages: Focus on spectral markers of collagen hydration. J. Raman Spectrosc. 2013, 44, 1230–1237. [Google Scholar] [CrossRef]
- Lopes, C.d.C.A.; Limirio, P.H.J.O.; Novais, V.R.; Dechichi, P. Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Rubio, L.; Vargas, A.; Rivera, P.; López-Gambero, A.J.; Tovar, R.; Christians, J.K.; Martín-de-las-Heras, S.; Rodríguez de Fonseca, F.; Chowen, J.A.; Argente, J.; et al. Recombinant IGF-1 Induces Sex-Specific Changes in Bone Composition and Remodeling in Adult Mice with Pappa2 Deficiency. Int. J. Mol. Sci. 2021, 22, 4048. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Mendelsohn, R.; Boskey, A.L. Infrared Assessment of Bone Quality: A Review. Clin. Orthop. Relat. Res. 2011, 469, 2170–2178. [Google Scholar] [CrossRef]
- Paschalis, E.P. Fourier Transform Infrared Imaging of Bone. In Bone Research Protocols; Idris, A.I., Ed.; Springer: New York, NY, USA, 2019; pp. 641–649. [Google Scholar]
- Taylor, E.A.; Lloyd, A.A.; Salazar-Lara, C.; Donnelly, E. Raman and Fourier Transform Infrared (FT-IR) Mineral to Matrix Ratios Correlate with Physical Chemical Properties of Model Compounds and Native Bone Tissue. Appl. Spectrosc. 2017, 71, 2404–2410. [Google Scholar] [CrossRef]
- Rubio, L.; Suárez, J.; Martin-de-las-Heras, S.; Zapico, S.C. Partners in Postmortem Interval Estimation: X-ray Diffraction and Fourier Transform Spectroscopy. Int. J. Mol. Sci. 2023, 24, 6793. [Google Scholar] [CrossRef]
- Vieira, A.L.; Nespeca, M.G.; Pavini, W.D.; Ferreira, E.C.; Gomes Neto, J.A. A user-friendly excel spreadsheet for dealing with spectroscopic and chromatographic data. Chemom. Intell. Lab. Syst. 2019, 194, 103816. [Google Scholar] [CrossRef]
- Buchwald, T.; Niciejewski, K.; Kozielski, M.; Szybowicz, M.; Siatkowski, M.; Krauss, H. Identifying compositional and structural changes in spongy and subchondral bone from the hip joints of patients with osteoarthritis using Raman spectroscopy. J. Biomed. Opt. 2012, 17, 017007. [Google Scholar] [CrossRef]
- Penel, G.; Delfosse, C.; Descamps, M.; Leroy, G. Composition of bone and apatitic biomaterials as revealed by intravital Raman microspectroscopy. Bone 2005, 36, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Kozielski, M.; Buchwald, T.; Szybowicz, M.; Blaszczak, Z.; Piotrowski, A.; Ciesielczyk, B. Determination of composition and structure of spongy bone tissue in human head of femur by Raman spectral mapping. J. Mater. Sci. Mater. Med. 2011, 22, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Kazanci, M.; Wagner, H.D.; Manjubala, N.I.; Gupta, H.S.; Paschalis, E.; Roschger, P.; Fratzl, P. Raman imaging of two orthogonal planes within cortical bone. Bone 2007, 41, 456–461. [Google Scholar] [CrossRef]
- Goodyear, S.R.; Gibson, I.R.; Skakle, J.M.; Wells, R.P.; Aspden, R.M. A comparison of cortical and trabecular bone from C57 Black 6 mice using Raman spectroscopy. Bone 2009, 44, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid. State. Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Ding, Y.; Leng, Y. Infrared spectroscopic characterization of carbonated apatite: A combined experimental and computational study. J. Biomed. Mater. Res. A 2014, 102, 496–505. [Google Scholar] [CrossRef]
- Brink, K.S.; Reisz, R.R.; LeBlanc, A.R.H.; Chang, R.S.; Lee, Y.C.; Chiang, C.C.; Huang, T.; Evans, D.C. Developmental and evolutionary novelty in the serrated teeth of theropod dinosaurs. Sci. Rep. 2015, 5, 12338. [Google Scholar] [CrossRef]
- Orilisi, G.; Monterubbianesi, R.; Notarstefano, V.; Tosco, V.; Vitiello, F.; Giuliani, G.; Putignano, A.; Orsini, G. New insights from Raman MicroSpectroscopy and Scanning Electron Microscopy on the microstructure and chemical composition of vestibular and lingual surfaces in permanent and deciduous human teeth. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 260, 119966. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Orilisi, G.; Notarstefano, V.; Monterubbianesi, R.; Vitiello, F.; Tosco, V.; Belloni, A.; Putignano, A.; Giorgini, E. Vibrational Imaging Techniques for the Characterization of Hard Dental Tissues: From Bench-Top to Chair-Side. Appl. Sci. 2021, 11, 11953. [Google Scholar] [CrossRef]
- Sadat-Ali, M.; al-Umran, K.; al-Habdan, I.; al-Mulhim, F. Ultrasonography: Can it differentiate between vasoocclusive crisis and acute osteomyelitis in sickle cell disease? J. Pediatr. Orthop. 1998, 18, 552–554. [Google Scholar] [CrossRef]
- Berger, E.; Saunders, N.; Wang, L.; Friedman, J.N. Sickle cell disease in children: Differentiating osteomyelitis from vaso-occlusive crisis. Arch. Pediatr. Adolesc. Med. 2009, 163, 251–255. [Google Scholar] [CrossRef]
- Le Blond, S.; Guilminot, E.; Lemoine, G.; Huet, N.; Mevellec, J.Y. FT-Raman spectroscopy: A positive means of evaluating the impact of whale bone preservation treatment. Vib. Spectrosc. 2009, 51, 156–161. [Google Scholar] [CrossRef]
- Goswami, K.; Parvizi, J.; Maxwell Courtney, P. Current Recommendations for the Diagnosis of Acute and Chronic PJI for Hip and Knee-Cell Counts, Alpha-Defensin, Leukocyte Esterase, Next-generation Sequencing. Curr. Rev. Musculoskelet. Med. 2018, 11, 428–438. [Google Scholar] [CrossRef]
- Omucheni, D.L.; Kaduki, K.A.; Bulimo, W.D.; Angeyo, H.K. Application of principal component analysis to multispectral-multimodal optical image analysis for malaria diagnostics. Malar. J. 2014, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.J.; German, M.J.; Singh, M.; Pollock, H.M.; Hammiche, A.; Kyrgiou, M.; Stringfellow, H.F.; Paraskevaidis, E.; Martin-Hirsch, P.L.; Martin, F.L. IR microspectroscopy: Potential applications in cervical cancer screening. Cancer Lett. 2007, 246, 1–11. [Google Scholar] [CrossRef]
- Eikje, N.S.; Aizawa, K.; Ozaki, Y. Vibrational spectroscopy for molecular characterisation and diagnosis of benign, premalignant and malignant skin tumours. Biotechnol. Annu. Rev. 2005, 11, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Desmedt, C.; Larsimont, D.; Sotiriou, C.; Goormaghtigh, E. Change in the microenvironment of breast cancer studied by FTIR imaging. Analyst 2013, 138, 4058–4065. [Google Scholar] [CrossRef] [PubMed]
- Pezzei, C.; Pallua, J.D.; Schaefer, G.; Seifarth, C.; Huck-Pezzei, V.; Bittner, L.K.; Klocker, H.; Bartsch, G.; Bonn, G.K.; Huck, C.W. Characterization of normal and malignant prostate tissue by Fourier transform infrared microspectroscopy. Mol. Biosyst. 2010, 6, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Pallua, J.D.; Pezzei, C.; Zelger, B.; Schaefer, G.; Bittner, L.K.; Huck-Pezzei, V.A.; Schoenbichler, S.A.; Hahn, H.; Kloss-Brandstaetter, A.; Kloss, F.; et al. Fourier transform infrared imaging analysis in discrimination studies of squamous cell carcinoma. Analyst 2012, 137, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Willenbacher, E.; Brunner, A.; Zelger, B.; Unterberger, S.H.; Stalder, R.; Huck, C.W.; Willenbacher, W.; Pallua, J.D. Application of mid-infrared microscopic imaging for the diagnosis and classification of human lymphomas. J. Biophotonics 2021, 14, e202100079. [Google Scholar] [CrossRef] [PubMed]
- Longato, S.; Woss, C.; Hatzer-Grubwieser, P.; Bauer, C.; Parson, W.; Unterberger, S.H.; Kuhn, V.; Pemberger, N.; Pallua, A.K.; Recheis, W.; et al. Post-mortem interval estimation of human skeletal remains by micro-computed tomography, mid-infrared microscopic imaging and energy dispersive X-ray mapping. Anal. Methods 2015, 7, 2917–2927. [Google Scholar] [CrossRef]
- Pallua, J.D.; Unterberger, S.H.; Pemberger, N.; Woess, C.; Ensinger, C.; Zelger, B.; Lass-Flörl, C.; Lackner, M. Retrospective case study on the suitability of mid-infrared microscopic imaging for the diagnosis of mucormycosis in human tissue sections. Anal. Methods 2017, 9, 4135–4142. [Google Scholar] [CrossRef]
- Woess, C.; Unterberger, S.H.; Roider, C.; Ritsch-Marte, M.; Pemberger, N.; Cemper-Kiesslich, J.; Hatzer-Grubwieser, P.; Parson, W.; Pallua, J.D. Assessing various Infrared (IR) microscopic imaging techniques for post-mortem interval evaluation of human skeletal remains. PLoS ONE 2017, 12, e0174552. [Google Scholar] [CrossRef]
- Talsma, D.T.; Ploegmakers, J.J.W.; Jutte, P.C.; Kampinga, G.; Wouthuyzen-Bakker, M. Time to positivity of acute and chronic periprosthetic joint infection cultures. Diagn. Microbiol. Infect. Dis. 2021, 99, 115178. [Google Scholar] [CrossRef] [PubMed]
- Ting, N.T.; Della Valle, C.J. Diagnosis of Periprosthetic Joint Infection-An Algorithm-Based Approach. J. Arthroplast. 2017, 32, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Bürger, J.; Palmowski, Y.; Strube, P.; Perka, C.; Putzier, M.; Pumberger, M. Low sensitivity of histopathological examination of peri-implant tissue samples in diagnosing postoperative spinal implant infection. Bone Jt. J. 2020, 102-b, 899–903. [Google Scholar] [CrossRef]
- Sousa, R.; Carvalho, A.; Santos, A.C.; Abreu, M.A. Optimal microbiological sampling for the diagnosis of osteoarticular infection. EFORT Open Rev. 2021, 6, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Liu, Q.; Guan, Z.; Liu, M.; Sun, X.; Zhu, X.; Chen, J.; Feng, W.; Li, J.; Zeng, J.; et al. Diagnostic accuracy of sonication fluid cultures from prosthetic components in periprosthetic joint infection: An updated diagnostic meta-analysis. J. Orthop. Surg. Res. 2023, 18, 175. [Google Scholar] [CrossRef]
- Bürger, J.; Akgün, D.; Strube, P.; Putzier, M.; Pumberger, M. Sonication of removed implants improves microbiological diagnosis of postoperative spinal infections. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2019, 28, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Kalbian, I.; Park, J.W.; Goswami, K.; Lee, Y.K.; Parvizi, J.; Koo, K.H. Culture-negative periprosthetic joint infection: Prevalence, aetiology, evaluation, recommendations, and treatment. Int. Orthop. 2020, 44, 1255–1261. [Google Scholar] [CrossRef]





| Age (Years) | Total Number with Gender (W = Female; M = Male) | Inoculation with Staph. epidermidis ATCC 12228 |
|---|---|---|
| >80 | W = 1 M = 1 | W = 0 M = 0 |
| 70–80 | W = 9 M = 7 | W = 1 M = 2 |
| 60–70 | W = 4 M = 5 | W = 1 M = 0 |
| 50–60 | W = 6 M = 2 | W = 3 M = 0 |
| <50 | W = 2 M = 3 | W = 2 M = 1 |
| Name | Description | p-Values (Two-Sample t-Test) | |
|---|---|---|---|
| Agilent 4300 | Perkin Elmer Spectrum 100 | ||
| Phosphate | ν3PO43− Amount of phosphate | 0.0021 | 0.1749 |
| Mineral/matrix (MMR) phosphate/amide I | ν3PO43−/amide I Mineral component amount to the organic one | 0.4428 | <0.0001 |
| Mineral quality and crystallinity carbonate/phosphate | ν1CO32−/ν1PO43− Carbonate incorporation extent in the hydroxyapatite lattice | 0.0620 | <0.0001 |
| Mineral carbonate content (MinCarb) | ν1CO32−/(C-H) bend; CH2 wag | 0.8890 | <0.0001 |
| Amide I | Amide I of α-helical structures Arrangement and quantity of collagen | 0.0032 | 0.0371 |
| CH-aliphatic content (CHACont) | CH2 stretching | 0.0080 | 0.6949 |
| Wave Number Range Number | PCA Perkin Elmer Spectrum 100 | PCA Agilent 4300 Handheld | Assignment | Spectral Region |
|---|---|---|---|---|
| I | PC-1 (73%) PC-2 (14%) | PC-1 (62%) PC-2 (21%) | Full wavenumber range | 650–4000 cm−1 |
| II | PC-1 (95%) PC-2 (5%) | PC-1 (97%) PC-2 (2%) | Amide III | 1200–1390 cm−1 |
| III | PC-1 (92%) PC-2 (5%) | PC-1 (64%) PC-2 (28%) | Amide III, CH2 deformation (wagging) of protein, amide I | 1200–1700 cm−1 |
| IV | PC-1 (100%) PC-2 (0%) | PC-1 (98%) PC-2 (2%) | Amide I | 1610–1700 cm−1 |
| V | PC-1 (91%) PC-2 (8%) | PC-1 (91%) PC-2 (8%) | C-H groups (bending and stretching modes) | 2800–3050 cm−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindtner, R.; Wurm, A.; Kugel, K.; Kühn, J.; Putzer, D.; Arora, R.; Coraça-Huber, D.C.; Zelger, P.; Schirmer, M.; Badzoka, J.; et al. Comparison of Mid-Infrared Handheld and Benchtop Spectrometers to Detect Staphylococcus epidermidis in Bone Grafts. Bioengineering 2023, 10, 1018. https://doi.org/10.3390/bioengineering10091018
Lindtner R, Wurm A, Kugel K, Kühn J, Putzer D, Arora R, Coraça-Huber DC, Zelger P, Schirmer M, Badzoka J, et al. Comparison of Mid-Infrared Handheld and Benchtop Spectrometers to Detect Staphylococcus epidermidis in Bone Grafts. Bioengineering. 2023; 10(9):1018. https://doi.org/10.3390/bioengineering10091018
Chicago/Turabian StyleLindtner, Richard, Alexander Wurm, Katrin Kugel, Julia Kühn, David Putzer, Rohit Arora, Débora Cristina Coraça-Huber, Philipp Zelger, Michael Schirmer, Jovan Badzoka, and et al. 2023. "Comparison of Mid-Infrared Handheld and Benchtop Spectrometers to Detect Staphylococcus epidermidis in Bone Grafts" Bioengineering 10, no. 9: 1018. https://doi.org/10.3390/bioengineering10091018