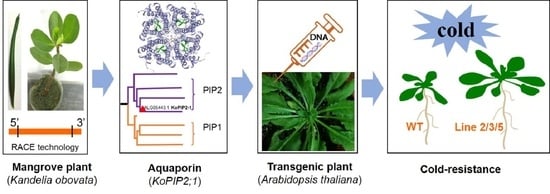
An Aquaporin Gene (KoPIP2;1) Isolated from Mangrove Plant Kandelia obovata Had Enhanced Cold Tolerance of Transgenic Arabidopsis thaliana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Treatments
2.2. Isolation of Total RNA and cDNA Synthesis
2.3. Cloning the Full-Length cDNA of KoPIP2;1 Gene
2.4. Bioinformatics Analysis of KoPIP2;1 Gene
2.5. Expression Analysis by RT-qPCR
2.6. Subcellular Localization Analysis
2.7. Generation of KoPIP2;1 Transgenic Arabidopsis Plants
2.8. Physiological Analysis of Transgenic A. thaliana Lines Exposed to Cold Stress
3. Results
3.1. Characterization and Sequence Analysis of KoPIP2;1
3.2. Phylogenic Analysis at the Protein Level
3.3. Expression Patterns of KoPIP2;1 in Response to Cold Stress
3.4. Subcellular Localization of KoPIP2;1 in Tobacco Epidermal Cells
3.5. Overexpression of KoPIP2;1 Enhanced Cold Tolerance of Transgenic Arabidopsis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shivaraj, S.M.; Sharma, Y.; Chaudhary, J.; Rajora, N.; Sharma, S.; Thakral, V.; Ram, H.; Sonah, H.; Singla-Pareek, S.L.; Sharma, T.R.; et al. Dynamic role of aquaporin transport system under drought stress in plants. Environ. Exp. Bot. 2021, 184, 104367. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.J.; Liu, F.; Sun, L.R.; Hao, F.S. Versatile roles of aquaporins in plant growth and development. Int. J. Mol. Sci. 2020, 21, 9485. [Google Scholar] [CrossRef] [PubMed]
- Noronha, H.; Silva, A.; Mitani-Ueno, N.; Conde, C.; Sabir, F.; Prista, C.; Soveral, G.; Isenring, P.; Ma, J.F.; Belanger, R.R.; et al. The grapevine NIP2;1 aquaporin is a silicon channel. J. Exp. Bot. 2020, 71, 6789–6798. [Google Scholar] [CrossRef] [PubMed]
- Scharwies, J.D.; Dinneny, J.R. Water transport, perception, and response in plants. J. Plant Res. 2019, 132, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Q.; You, S.Y.; Wang, Y.B.; Huang, L.; Wang, M. Influence of frost on nutrient resorption during leaf senescence in a mangrove at its latitudinal limit of distribution. Plant Soil 2011, 342, 105–115. [Google Scholar] [CrossRef]
- Cheng, X.F.; Wu, H.H.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizal response strategies of trifoliate orange under well-watered, salt stress, and waterlogging stress by regulating leaf aquaporin expression. Plant Physiol. Bioch. 2021, 162, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, W.; Liu, J.H.; Song, S.; Hou, X.W.; Jia, C.H.; Li, J.Y.; Miao, H.X.; Wang, Z.; Tie, W.W.; et al. An aquaporin gene MaPIP2-7 is involved in tolerance to drought, cold and salt stresses in transgenic banana (Musa acuminata L.). Plant Physiol. Biochem. 2020, 147, 66–76. [Google Scholar] [CrossRef]
- Hussain, A.; Tanveer, R.; Mustafa, G.; Farooq, M.; Amin, I.; Mansoor, S. Comparative phylogenetic analysis of aquaporins provides insight into the gene family expansion and evolution in plants and their role in drought tolerant and susceptible chickpea cultivars. Genomics 2020, 112, 263–275. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Fischer, M. Functional aquaporin diversity in plants. BBA Biomembr. 2006, 1758, 1134–1141. [Google Scholar] [CrossRef] [Green Version]
- Heymann, J.B.; Engel, A. Structural clues in the sequences of the aquaporins. J. Mol. Biol. 2000, 295, 1039–1053. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, R.; Ren, T.; Shankla, M.; Decker, K.; Grisewood, M.; Prabhakar, J.; Baker, C.; Golbeck, J.H.; Aksimentiev, A.; Kumar, M.; et al. PoreDesigner for tuning solute selectivity in a robust and highly permeable outer membrane pore. Nat. Commun. 2018, 9, 3661. [Google Scholar] [CrossRef] [Green Version]
- Törnroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef]
- Zhang, W.E.; Hu, J.M.; Li, F.; Chen, E.J.; Zhao, T.; Pan, X.J. Cloning and expression of tonoplast membrane intrinsic protein genes in leaves of Vitis heyneana and overexpression of VhTIP2;1 in Arabidopsis confer drought tolerance. Acta Physiol. Plant 2023, 45, 44. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.W.; Ji, C.C.; Wei, Z.X.; Zhao, T.; Pang, Q.Y. Overexpression of an aquaporin gene EsPIP1;4 enhances abiotic stress tolerance and promotes flowering in Arabidopsis thaliana. Plant Physiol. Bioch. 2022, 193, 25–35. [Google Scholar] [CrossRef]
- Aharon, R.; Shahak, Y.; Wininger, S.; Bendov, R.; Kapulnik, Y.; Galili, G. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 2003, 15, 439–447. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, S.H.; Rhee, J.Y.; Chung, G.C.; Ahn, S.J.; Kang, H. Transgenic Arabidopsis and tobacco plants overexpressing an aquaporin respond differently to various abiotic stresses. Plant Mol. Biol. 2007, 64, 621–632. [Google Scholar] [CrossRef]
- Tregarot, E.; Caillaud, A.; Cornet, C.C.; Taureau, F.; Catry, T.; Cragg, S.M.; Failler, P. Mangrove ecological services at the forefront of coastal change in the French overseas territories. Sci. Total Environ. 2021, 763, 143004. [Google Scholar] [CrossRef]
- Chen, L.Z.; Wang, W.Q.; Zhang, Y.H.; De, Y.H.; Huang, L.; Zhao, C.L.; Yang, S.C.; Yang, Z.W.; Chen, Y.C.; Xu, H.L.; et al. Damage to mangroves from extreme cold in early 2008 in Southern China. Acta Phytoecol. Sin. 2010, 34, 186–194. [Google Scholar]
- Lu, W.X.; Zhang, B.H.; Yang, S.C. Survive the north: Transplantation for conservation of mangrove forests requires consideration of influences of low temperature, mating system and their joint effects on effective size of the reforested populations. Front. Ecol. Evol. 2023, 11, 1160468. [Google Scholar] [CrossRef]
- Sohag, A.M.; Tahjib-Ul-Arif, M.; Afrin, S.; Khan, M.K.; Hannan, A.; Skalicky, M.; Mortuza, M.G.; Brestic, M.; Hossain, M.A.; Murata, Y. Insights into nitric oxide-mediated water balance, antioxidant defence and mineral homeostasis in rice (Oryza sativa L.) under chilling stress. Nitric Oxide-Biol. Chem. 2020, 100, 7–16. [Google Scholar] [CrossRef]
- Tian, K.; Li, Q.; Zhang, X.M.; Guo, H.Y.; Wang, Y.H.; Cao, P.L.; Xu, S.Y.; Li, W.Y. Analysis of the expression and function of the CBL-CIPK network and MAPK cascade genes in Kandelia obovata seedlings under cold stress. Front. Mar. Sci. 2023, 10, 1113278. [Google Scholar] [CrossRef]
- Ozu, M.; Alvear-Arias, J.J.; Fernandez, M.; Caviglia, A.; Pena-Pichicoi, A.; Carrillo, C.; Carmona, E.; Otero-Gonzalez, A.; Garate, J.A.; Amodeo, G.; et al. Aquaporin gating: A new twist to unravel permeation through water channels. Int. J. Mol. Sci. 2022, 23, 12317. [Google Scholar] [CrossRef]
- Zhao, C.X.; Shao, H.B.; Chu, L.Y. Aquaporin structure-function relationships, water flow through plant living cells. Colloid Surf. B 2008, 62, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Fang, X.D.; Lin, Q.F.; Li, G.Y.; Zhao, W.M. Identification and expression analysis of a full-length cDNA encoding a Kandelia candel tonoplast intrinsic protein. Act. J. Pharm. Biotech. 2003, 19, 147–152. [Google Scholar]
- Lu, Y.J.; Han, Y.S.; Li, N.N.; Hou, P.C.; Huang, X.X.; Deng, S.R.; Zhao, R.; Shen, X.; Chen, S.L. Molecular cloning and salt-tolerance of gene KcTIP1 from Kandelia candel. J. Northwest Agric. For. Univ. China 2013, 41, 162–171. [Google Scholar]
- Reef, R.; Schmitz, N.; Rogers, B.A.; Ball, M.C.; Lovelock, C.E. Differential responses of the mangrove Avicennia marina to salinity and abscisic acid. Funct. Plant Biol. 2012, 39, 1038–1046. [Google Scholar] [CrossRef]
- Tan, W.K.; Lin, Q.; Lim, T.M.; Kumar, P.; Loh, C.S. Dynamic secretion changes in the salt glands of the mangrove tree species Avicennia officinalis, in response to a changing saline environment. Plant Cell Environ. 2013, 36, 1410–1422. [Google Scholar] [CrossRef]
- Fei, J.; Wang, Y.S.; Jiang, Z.Y.; Cheng, H.; Zhang, J.D. Identification of cold tolerance genes from leaves of mangrove plant Kandelia obovata by suppression subtractive hybridization. Ecotoxicology 2015, 24, 1686–1696. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.S. Analysis and improvement of high-quality RNA extraction in leaves of mangrove plants. Ecol. Sci. 2011, 30, 201–206. [Google Scholar]
- Fei, J.; Wang, Y.S.; Cheng, H.; Su, Y.B.; Zhong, Y.J.; Zheng, L. Cloning and characterization of KoOsmotin from mangrove plant Kandelia obovata under cold stress. BMC Plant Biol. 2021, 21, 10. [Google Scholar] [CrossRef]
- Peng, Y.L.; Wang, Y.S.; Cheng, H.; Sun, C.C.; Wu, P.; Wang, L.Y.; Fei, J. Characterization and expression analysis of three CBF/DREB1 transcriptional factor genes from mangrove Avicennia marina. Aquat. Toxicol. 2013, 140, 68–76. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lei, X.J.; Tan, B.; Liu, Z.Y.; Wu, J.; Lv, J.X.; Gao, C.Q. ThCOL2 improves the salt stress tolerance of Tamarix hispida. Front. Plant Sci. 2021, 12, 653791. [Google Scholar] [CrossRef]
- Bechtold, N.; Pelletier, G. In planta Agrobacterium mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 1998, 82, 259–266. [Google Scholar]
- Weig, A.; Deswarte, C.; Chrispeels, M.J. The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol. 1997, 114, 1347–1357. [Google Scholar] [CrossRef] [Green Version]
- Frick, A.; Jarva, M.; Ekvall, M.; Uzdavinys, P.; Nyblom, M.; Tornroth-Horsefield, S. Mercury increases water permeability of a plant aquaporin through a non-cysteine-related mechanism. Biochem. J. 2013, 454, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Fei, J.; Wang, Y.S.; Cheng, H.; Su, Y.B.; Zhong, Y.J.; Zheng, L. The Kandelia obovata transcription factor KoWRKY40 enhances cold tolerance in transgenic Arabidopsis. BMC Plant Biol. 2022, 22, 274. [Google Scholar] [CrossRef]
- Bienert, M.D.; Diehn, T.A.; Richet, N.; Chaumont, F.; Bienert, G.P. Heterotetramerization of plant PIP1 and PIP2 aquaporins is an evolutionary ancient feature to guide PIP1 plasma membrane localization and function. Front. Plant Sci. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.F.; Li, R.; Li, D.B.; Xu, F.F.; Sun, Q.Q.; Zhao, B.; Mao, A.J.; Guo, Y.D. Expression patterns of genes encoding plasma membrane aquaporins during fruit development in cucumber (Cucumis sativus L.). Plant Physiol. Bioch. 2015, 96, 329–336. [Google Scholar] [CrossRef]
- Kumawat, S.; Khatri, P.; Ahmed, A.; Vats, S.; Kumar, V.; Jaswal, R.; Wang, Y.; Xu, P.; Mandlik, R.; Shivaraj, S.M.; et al. Understanding aquaporin transport system, silicon and other metalloids uptake and deposition in bottle gourd (Lagenaria siceraria). J. Hazard Mater. 2021, 409, 124598. [Google Scholar] [CrossRef]
- Jia, J.H.; Liang, Y.F.; Gou, T.Y.; Hu, Y.H.; Zhu, Y.X.; Hu, H.Q.; Guo, J.; Gong, H.J. The expression response of plasma membrane aquaporins to salt stress in tomato plants. Environ. Exp. Bot. 2020, 178, 104190. [Google Scholar] [CrossRef]
- Tailor, A.; Bhatla, S.C. Polyamine homeostasis modulates plasma membrane- and tonoplast-associated aquaporin expression in etiolated salt-stressed sunflower (Helianthus annuus L.) seedlings. Protoplasma 2021, 258, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.F.; Xu, H.; Khan, S.; Equiza, M.A.; Lee, S.H.; Vaziriyeganeh, M.; Zwiazek, J.J. Plant water transport and aquaporins in oxygen-deprived environments. J. Plant Physiol. 2018, 227, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qiang, X.J.; Han, X.R.; Jiang, L.L.; Zhang, S.H.; Han, J.; He, R.; Cheng, X.G. Ectopic expression of a Thellungiella salsuginea aquaporin gene, TsPIP1;1, increased the salt tolerance of rice. Int. J. Mol. Sci. 2018, 19, 2229. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, C.; Wang, T.; Guo, Z.; Lu, S. Overexpression of MfPIP2-7 from Medicago falcata promotes cold tolerance and growth under NO3− deficiency in transgenic tobacco plants. BMC Plant Biol. 2016, 16, 138. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.Y.; Liu, X.S.; Khan, I.U.; Wu, X.C.; Yang, Z.M. OsPIP2;3 as an aquaporin contributes to rice resistance to water deficit but not to salt stress. Environ. Exp. Bot. 2021, 183, 104342. [Google Scholar] [CrossRef]
- Peng, Y.H.; Lin, W.L.; Cai, W.M.; Arora, R. Overexpression of a Panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic Arabidopsis plants. Planta 2007, 226, 729–740. [Google Scholar] [CrossRef]
- Li, J.; Xia, W.W.; Zang, H.X.; Dai, B.; Zhang, Y.; Feng, Y.J.; Wang, A.Y.; Lin, Z.P.; Liu, H.L.; Zhu, J.B. Expression analysis of aquaporin genes in Saussurea involucrata rosette leaves and functional analysis of upregulated SiPIP1;5A under low-temperature stress. Environ. Exp. Bot. 2020, 171, 103958. [Google Scholar] [CrossRef]
- Koenigshofer, H.; Loeppert, H.G. The up-regulation of proline synthesis in the meristematic tissues of wheat seedlings upon short-term exposure to osmotic stress. J. Plant Physiol. 2019, 237, 21–29. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Chomkitichai, W.; Chumyam, A.; Rachtanapun, P.; Uthaibutra, J.; Saengnil, K. Reduction of reactive oxygen species production and membrane damage during storage of ‘Daw’ longan fruit by chlorine dioxide. Sci. Hortic. 2014, 170, 143–149. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, W.; Liu, J.; Zhang, J.; Jia, C.; Miao, H.; Xu, B.; Jin, Z. A banana aquaporin gene, MaPIP1;1, is involved in tolerance to drought and salt stresses. BMC Plant Biol. 2014, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Wen, M.; Lin, X.; Yu, Y.S.; Wu, J.J.; Xu, Y.J.; Xiao, G.S. Natamycin treatment reduces the quality changes of postharvest mulberry fruit during storage. J. Food Biochem. 2019, 43, e12934. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, J.; Wang, Y.; Cheng, H.; Wang, H.; Wu, M.; Sun, F.; Sun, C. An Aquaporin Gene (KoPIP2;1) Isolated from Mangrove Plant Kandelia obovata Had Enhanced Cold Tolerance of Transgenic Arabidopsis thaliana. Bioengineering 2023, 10, 878. https://doi.org/10.3390/bioengineering10070878
Fei J, Wang Y, Cheng H, Wang H, Wu M, Sun F, Sun C. An Aquaporin Gene (KoPIP2;1) Isolated from Mangrove Plant Kandelia obovata Had Enhanced Cold Tolerance of Transgenic Arabidopsis thaliana. Bioengineering. 2023; 10(7):878. https://doi.org/10.3390/bioengineering10070878
Chicago/Turabian StyleFei, Jiao, Youshao Wang, Hao Cheng, Hui Wang, Meilin Wu, Fulin Sun, and Cuici Sun. 2023. "An Aquaporin Gene (KoPIP2;1) Isolated from Mangrove Plant Kandelia obovata Had Enhanced Cold Tolerance of Transgenic Arabidopsis thaliana" Bioengineering 10, no. 7: 878. https://doi.org/10.3390/bioengineering10070878