Scalable and High-Throughput In Vitro Vibratory Platform for Vocal Fold Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
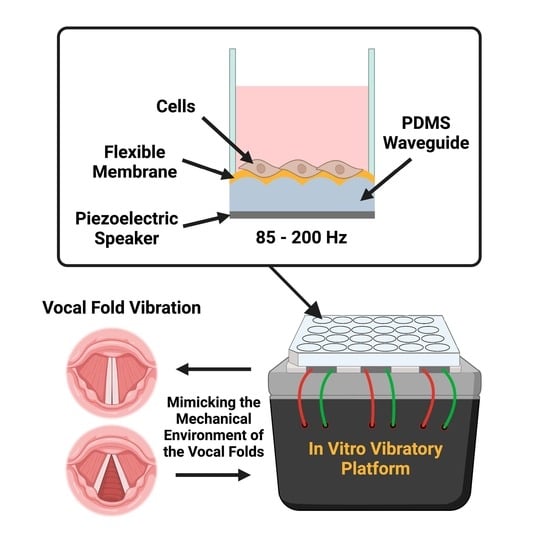
2.1. Platform Fabrication and Assembly
2.2. Laser Doppler Vibrometry (LDV) Measurements
2.3. Cell Culture Conditions
2.4. Gene Expression Analysis
2.5. Statistical Analysis
3. Results
3.1. Vibratory Platform Model
3.2. Platform Characterization
3.3. Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Svec, J.G.; Granqvist, S. Guidelines for Selecting Microphones for Human Voice Production Research. Am. J. Speech-Lang. Pathol. Am. Speech-Lang.-Hear. Assoc. 2010, 19, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K. Mechanical Characterization of Vocal Fold Tissue: A Review Study. J. Voice 2014, 28, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Li, N. Current Understanding and Future Directions for Vocal Fold Mechanobiology. J. Cytol. Mol. Biol. 2013, 1, 001. [Google Scholar] [CrossRef] [PubMed]
- Brunkan, M.C. Preservice Music Teacher Voice Use, Vocal Health, and Voice Function Before and During Student Teaching. J. Music Teach. Educ. 2018, 27, 80–93. [Google Scholar] [CrossRef]
- Jiang, J.; Bless, D. Vocal Fold Stress in Voice Overuse and Abuse. Sig 3 Perspect. Voice Voice Disord. 2003, 13, 3–6. [Google Scholar] [CrossRef]
- Choi, S.H.; Choi, C.-H. The Characteristics of Voice Handicap Index and Vocal Misuse and Overuse in Female Elementary Teachers. J. Korean Soc. Speech Sci. 2013, 5, 53–61. [Google Scholar] [CrossRef]
- Franco, R.A.; Andrus, J.G. Common Diagnoses and Treatments in Professional Voice Users. Otolaryngol. Clin. N. Am. 2007, 40, 1025–1061. [Google Scholar] [CrossRef]
- Duarte, J.L.; de Faria, F.A.C.; Ceolin, D.S.; Cestari, T.M.; de Assis, G.F. Effects of Passive Smoke Inhalation on the Vocal Cords of Rats. Braz. J. Otorhinolaryngol. 2006, 72, 210–216. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, W.; Sivasankar, M.P. Acute Acrolein Exposure Induces Impairment of Vocal Fold Epithelial Barrier Function. PLoS ONE 2016, 11, e0163237. [Google Scholar] [CrossRef]
- Cherian, A.; Balachander, H.; Nagappa, M.; Rajagopal, V. Bilateral Vocal Cord Palsy Following Endotracheal Intubation: A Case Report. J. Clin. Anesth. 2010, 22, 562–564. [Google Scholar] [CrossRef]
- House, J.C.; Noordzij, J.P.; Murgia, B.; Langmore, S. Laryngeal Injury from Prolonged Intubation: A Prospective Analysis of Contributing Factors. Laryngoscope 2011, 121, 596–600. [Google Scholar] [CrossRef]
- Sataloff, R.T.; Hawkshaw, M.; Hoover, C.; Spiegel, J.R. Post-Intubation Vocal Fold Scar. Ear Nose Throat J. 1997, 76, 128. [Google Scholar] [CrossRef]
- David, D.S.; Shah, M. Vocal Cord Paralysis Following Intubation. JAMA 1971, 216, 1645–1646. [Google Scholar] [CrossRef] [PubMed]
- Latoo, M.; Lateef, M.; Nawaz, I.; Ali, I. Bilateral Recurrent Laryngeal Nerve Palsy Following Blunt Neck Trauma. Indian J. Otolaryngol. 2007, 59, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Tran, Y.; Shah, A.K.; Mittal, S. Lead Breakage and Vocal Cord Paralysis Following Blunt Neck Trauma in a Patient with Vagal Nerve Stimulator. J. Neurol. Sci. 2011, 304, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Bilateral Vocal Cord Paralysis after Anterior Cervical Discectomy Following Cervical Spine Injury: A Case Report—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4771242/ (accessed on 17 July 2022).
- Tanigami, Y.; Kawai, Y.; Kaba, S.; Uozumi, R.; Ohnishi, H.; Kita, T.; Omori, K.; Kishimoto, Y. Establishment of a Radiation-Induced Vocal Fold Fibrosis Mouse Model. Biochem. Biophys. Res. Commun. 2022, 601, 31–37. [Google Scholar] [CrossRef]
- Chung, J.H.; Tae, K.; Lee, Y.S.; Jeong, J.H.; Cho, S.H.; Kim, K.R.; Park, C.W.; Han, D.S. The Significance of Laryngopharyngeal Reflux in Benign Vocal Mucosal Lesions. Otolaryngol. Head Neck Surg. 2009, 141, 369–373. [Google Scholar] [CrossRef]
- Wang, L.; Tan, J.-J.; Wu, T.; Zhang, R.; Wu, J.-N.; Zeng, F.-F.; Liu, Y.-L.; Han, X.-Y.; Li, Y.-F.; Li, X.-P. Association between Laryngeal Pepsin Levels and the Presence of Vocal Fold Polyps. Otolaryngol. Head Neck Surg. 2016, 156, 144–151. [Google Scholar] [CrossRef]
- Cobzeanu, M.D.; Drug, V.; Palade, O.D.; Ciobotaru, A.; Voineag, M. The Role of the Environmental Factors and Gastro-Esophageal Reflux in Vocal Professionals’ Dysphonia. In Proceedings of the 2012 International Conference and Exposition on Electrical and Power Engineering, Iasi, Romania, 25–27 October 2012; pp. 645–649. [Google Scholar] [CrossRef]
- Loor, A.C.; Nedelcuţ, S.; Dumitraşcu, D.L. High Prevalence of Gastroesophageal Reflux in Vocal Opera Students. A Case-Control Type Study. Med. Pharm Rep. 2020, 93, 145–149. [Google Scholar] [CrossRef]
- Beaver, M.E.A.; Kaszuba, S.M.; Stewart, M.G.; Stasney, C.R. Vocal Quality of Life Improves with Treatment of Laryngopharyngeal Reflux. Otolaryngol. Head Neck Surg. 2004, 131, P213. [Google Scholar] [CrossRef]
- Henry, M.A.C.d.A.; Martins, R.H.G.; Lerco, M.M.; Carvalho, L.R.; Lamônica-Garcia, V.C. Gastroesophageal Reflux Disease and Vocal Disturbances. Arq. Gastroenterol. 2011, 48, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.R.; Behlau, M. The Influence of Sleep Disorders on Voice Quality. J. Voice 2018, 32, 771.e1–771.e13. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stiadle, J.M.; Lau, H.K.; Zerdoum, A.B.; Jia, X.; Thibeault, S.L.; Kiick, K.L. Tissue Engineering-Based Therapeutic Strategies for Vocal Fold Repair and Regeneration. Biomaterials 2016, 108, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.G.; Biehl, A.; Sze, D.; Freytes, D.O. Bioreactors for Vocal Fold Tissue Engineering. Tissue Eng. Part B Rev. 2021, 28, 182–205. [Google Scholar] [CrossRef]
- Ling, C.; Li, Q.; Brown, M.E.; Kishimoto, Y.; Toya, Y.; Devine, E.E.; Choi, K.-O.; Nishimoto, K.; Norman, I.G.; Tsegyal, T.; et al. Bioengineered Vocal Fold Mucosa for Voice Restoration. Sci. Transl. Med. 2015, 7, 314ra187. [Google Scholar] [CrossRef]
- Bartlett, R.S.; Thibeault, S.L.; Prestwich, G.D. Therapeutic Potential of Gel-Based Injectables for Vocal Fold Regeneration. Biomed. Mater. 2012, 7, 024103. [Google Scholar] [CrossRef]
- Bartlett, R.S.; Gaston, J.D.; Yen, T.Y.; Ye, S.; Kendziorski, C.; Thibeault, S.L. Biomechanical Screening of Cell Therapies for Vocal Fold Scar. Tissue Eng. Part A 2015, 21, 2437–2447. [Google Scholar] [CrossRef]
- Branski, R.C.; Bing, R.; Kraja, I.; Amin, M.R. The Role of Smad3 in the Fibrotic Phenotype in Human Vocal Fold Fibroblasts. Laryngoscope 2015, 126, 1151–1156. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Gama, A.C.C.; Magalhães, M.D.C. Fundamental Voice Frequency: Acoustic, Electroglottographic, and Accelerometer Measurement in Individuals With and Without Vocal Alteration. J. Voice 2021, 35, 174–180. [Google Scholar] [CrossRef]
- Lamprecht, R.; Maghzinajafabadi, M.; Semmler, M.; Sutor, A. Imaging the Vocal Folds: A Feasibility Study on Strain Imaging and Elastography of Porcine Vocal Folds. Appl. Sci. 2019, 9, 2729. [Google Scholar] [CrossRef]
- Chan, R.W.; Fu, M.; Young, L.; Tirunagari, N. Relative Contributions of Collagen and Elastin to Elasticity of the Vocal Fold Under Tension. Ann. Biomed. Eng. 2007, 35, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Teller, S.S.; Farran, A.J.E.; Xiao, L.; Jiao, T.; Duncan, R.L.; Clifton, R.J.; Jia, X. High-Frequency Viscoelastic Shear Properties of Vocal Fold Tissues: Implications for Vocal Fold Tissue Engineering. Tissue Eng. Part A 2012, 18, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, S.; Lim, J.; Kwon, S. Characteristics and Responses of Human Vocal Fold Cells in a Vibrational Culture Model. Laryngoscope 2018, 128, E258–E264. [Google Scholar] [CrossRef]
- Kirsch, A.; Hortobagyi, D.; Stachl, T.; Karbiener, M.; Grossmann, T.; Gerstenberger, C.; Gugatschka, M. Development and Validation of a Novel Phonomimetic Bioreactor. PLoS ONE 2019, 14, e0213788. [Google Scholar] [CrossRef]
- Tong, Z.; Duncan, R.L.; Jia, X. Modulating the Behaviors of Mesenchymal Stem Cells Via the Combination of High-Frequency Vibratory Stimulations and Fibrous Scaffolds. Tissue Eng. Part A 2013, 19, 1862–1878. [Google Scholar] [CrossRef]
- Farran, A.J.E.; Teller, S.S.; Jia, F.; Clifton, R.J.; Duncan, R.L.; Jia, X. Design and Characterization of a Dynamic Vibrational Culture System. J. Tissue Eng. Regen. Med. 2013, 7, 213–225. [Google Scholar] [CrossRef]
- Titze, I.R.; Hitchcock, R.W.; Broadhead, K.; Webb, K.; Li, W.; Gray, S.D.; Tresco, P.A. Design and Validation of a Bioreactor for Engineering Vocal Fold Tissues under Combined Tensile and Vibrational Stresses. J. Biomech. 2004, 37, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Kutty, J.K.; Webb, K. Vibration Stimulates Vocal Mucosa-like Matrix Expression by Hydrogel-encapsulated Fibroblasts. J. Tissue Eng. Regen. Med. 2010, 4, 62–72. [Google Scholar] [CrossRef]
- Kim, D.; Lim, J.-Y.; Kwon, S. Development of Vibrational Culture Model Mimicking Vocal Fold Tissues. Ann. Biomed. Eng. 2016, 44, 3136–3143. [Google Scholar] [CrossRef]
- Branski, R.C.; Perera, P.; Verdolini, K.; Rosen, C.A.; Hebda, P.A.; Agarwal, S. Dynamic Biomechanical Strain Inhibits IL-1β–Induced Inflammation in Vocal Fold Fibroblasts. J. Voice 2007, 21, 651–660. [Google Scholar] [CrossRef]
- Tong, Z.; Zerdoum, A.B.; Duncan, R.L.; Jia, X. Dynamic Vibration Cooperates with Connective Tissue Growth Factor to Modulate Stem Cell Behaviors. Tissue Eng. Part A 2014, 20, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Zerdoum, A.B.; Tong, Z.; Bachman, B.; Jia, X. Construction and Characterization of a Novel Vocal Fold Bioreactor. J. Vis. Exp. 2014, e51594. [Google Scholar] [CrossRef]
- Zerdoum, A.B.; Saberi, P.; Stuffer, A.J.; Kelly, D.J.; Duncan, R.L.; Mongeau, L.; Jia, X. Regulation of Stem Cell Function in an Engineered Vocal Fold-Mimetic Environment. Regen. Eng. Transl. Med. 2020, 6, 164–178. [Google Scholar] [CrossRef] [PubMed]





| Measurement Mode | FFT (Frequency Domain) |
|---|---|
| Averaging | 3 Times |
| Sample Frequency | 25 kHz |
| Bandwidth | 0–1.25 kHz |
| Sample Time | 1.28 s |
| Resolution | 781.25 mHz |
| Speckle Tracking | Best |
| Vibrometer Controller (Mode) | 3D |
| Vibrometer Velocity | 500 mm/s |
| Vibrometer Tracking Filter | Slow |
| Number of Scan Points per Well | 33 Nodes |
| Nodal Mesh Radial Density | 1.75 mm |
| Nodal Mesh Angular Density | 45 Degrees |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | AAGGTGAAGGTCGGAGTCAAC | GGGGTCATTGATGGCAACAATA |
| ACTA2 | CCAGCAGATGTGGATCAGCAAACA | ACGAGTCAGAGCTTTGGCTAGGAA |
| MMP1 | CTCTGGAGTAATGTCACACCTCT | TGTTGGTCCACCTTTCATCTTC |
| HAS1 | GAGCCTCTTCGCGTACCTG | CCTCCTGGTAGGCGGAGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biehl, A.; Colmon, R.; Timofeeva, A.; Gracioso Martins, A.M.; Dion, G.R.; Peters, K.; Freytes, D.O. Scalable and High-Throughput In Vitro Vibratory Platform for Vocal Fold Tissue Engineering Applications. Bioengineering 2023, 10, 602. https://doi.org/10.3390/bioengineering10050602
Biehl A, Colmon R, Timofeeva A, Gracioso Martins AM, Dion GR, Peters K, Freytes DO. Scalable and High-Throughput In Vitro Vibratory Platform for Vocal Fold Tissue Engineering Applications. Bioengineering. 2023; 10(5):602. https://doi.org/10.3390/bioengineering10050602
Chicago/Turabian StyleBiehl, Andreea, Ramair Colmon, Anastasia Timofeeva, Ana Maria Gracioso Martins, Gregory R. Dion, Kara Peters, and Donald O. Freytes. 2023. "Scalable and High-Throughput In Vitro Vibratory Platform for Vocal Fold Tissue Engineering Applications" Bioengineering 10, no. 5: 602. https://doi.org/10.3390/bioengineering10050602