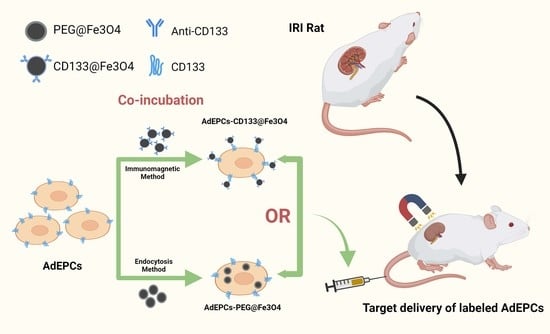
Immunomagnetic Delivery of Adipose-Derived Endothelial Progenitor Cells for the Repair of Renal Ischemia–Reperfusion Injury in a Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of MNPs
2.2. Animals and AdEPCs Isolation
2.3. Identification of AdEPCs
2.4. Magnetization of AdEPCs with MNPs
2.5. Examination of AdEPCs Magnetization
2.6. Cell Apoptosis Assay
2.7. Cell Proliferation Assay
2.8. Detection of ROS Levels
2.9. Migration Assay
2.10. Tube Formation Assay
2.11. Rat Renal IRI Model
2.12. Cell Tracking
2.13. Renal Function Analysis
2.14. Histological and Immunohistochemical Examination
2.15. HUVECs and AdEPCs Co-Culture Model
2.16. Statistical Consideration
3. Results
3.1. Isolation and Identification of AdEPCs
3.2. Characterization of PEG@Fe3O4 and CD133@Fe3O4
3.3. AdEPCs Magnetization with PEG@Fe3O4 and CD133@Fe3O4
3.4. The Influences of PEG@Fe3O4 and CD133@Fe3O4 on the Function of AdEPCs
3.5. In Vivo Tracking of AdEPCs
3.6. Outcomes of Renal Function and Tubular Damage
3.7. Cell Apoptosis and Proliferation, and Microvasculature in IRI Kidneys
3.8. The Effects of CD133+ AdEPCs on H/R HUVECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Renal. Inj. Prev. 2015, 4, 20–27. [Google Scholar]
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019, 96, 291–301. [Google Scholar] [CrossRef]
- Grams, M.E.; Sang, Y.; Coresh, J.; Ballew, S.; Matsushita, K.; Molnar, M.Z.; Szabo, Z.; Kalantar-Zadeh, K.; Kovesdy, C.P. Acute kidney injury after major surgery: A retrospective analysis of veterans health administration data. Am. J. Kidney Dis. 2016, 67, 872–880. [Google Scholar] [CrossRef] [PubMed]
- See, E.J.; Jayasinghe, K.; Glassford, N.; Bailey, M.; Johnson, D.W.; Polkinghorne, K.R.; Toussaint, N.D.; Bellomo, R. Long-term risk of adverse outcomes after acute kidney injury: A systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019, 95, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kim, D.-I.; Abbasi, S.A.; Latifi Gharamaleki, N.; Kim, E.; Jin, C.; Kim, S.; Hwang, J.; Kim, J.-Y.; Chen, X.-Z.; et al. Multi-target cell therapy using a magnetoelectric microscale biorobot for targeted delivery and selective differentiation of SH-SY5Y cells magnetically driven cell stamping. Mater. Horiz. 2022, 9, 3031–3038. [Google Scholar] [CrossRef]
- Wang, L.L.-W.; Janes, M.E.; Kumbhojkar, N.; Kapate, N.; Clegg, J.R.; Prakash, S.; Heavey, M.K.; Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Cell therapies in the clinic. Bioeng. Transl. Med. 2021, 6, e10214. [Google Scholar] [CrossRef]
- Soto, P.A.; Vence, M.; Pinero, G.M.; Coral, D.F.; Usach, V.; Muraca, D.; Cueto, A.; Roig, A.; van Raap, M.B.F.; Setton-Avruj, C.P. Sciatic nerve regeneration after traumatic injury using magnetic targeted adipose-derived mesenchymal stem cells. Acta Biomater. 2021, 130, 234–247. [Google Scholar] [CrossRef]
- Shen, W.-C.; Chou, Y.-H.; Huang, H.-P.; Sheen, J.-F.; Hung, S.-C.; Chen, H.-F. Induced pluripotent stem cell-derived endothelial progenitor cells attenuate ischemic acute kidney injury and cardiac dysfunction. Stem Cell Res. Ther. 2018, 9, 344. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Gatti, S.; Medica, D.; Figliolini, F.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia–reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012, 82, 412–427. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, K.; Wang, L.; Lu, T.; Zhou, C.; Ge, Y.; Wu, R.; Jia, R.; Zheng, C. Erythropoietin preconditioning mobilizes endothelial progenitor cells to attenuate nephron-sparing surgery-induced ischemia-reperfusion injury. Transplant. Proc. 2020, 52, 2955–2963. [Google Scholar] [CrossRef]
- Patschan, D.; Backhaus, R.; Elle, H.-J.; Schwarze, K.; Henze, E.; Becker, J.U.; Patschan, S.; Müller, G.A. Angiopoietin-2 modulates eEOC-mediated renoprotection in AKI in a dose-dependent manner. J. Nephrol. 2013, 26, 667–674. [Google Scholar] [CrossRef]
- Patschan, D.; Schwarze, K.; Henze, E.; Patschan, S.; Scheidemann, R.; Müller, G.A. Fibrate treatment of eEOCs in murine AKI. J. Nephrol. 2014, 27, 37–44. [Google Scholar] [CrossRef]
- Patschan, D.; Schwarze, K.; Lange, A.; Meise, N.; Henze, E.; Becker, J.U.; Patschan, S.; Müller, G.A. Bone morphogenetic protein-5 and early endothelial outgrowth cells (eEOCs) in acute ischemic kidney injury (AKI) and 5/6-chronic kidney disease. Am. J. Physiol. Renal. Physiol. 2013, 305, F314–F322. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Xu, L.; Qian, Y.; Liu, N.; Zhou, C.; Liu, J.; Zhou, L.; Xu, Z.; Jia, P. Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment. Stem Cell Res. Ther. 2022, 13, 238. [Google Scholar] [CrossRef]
- Zhou, L.; Xia, J.; Qiu, X.; Wang, P.; Jia, R.; Chen, Y.; Yang, B.; Dai, Y. In vitro evaluation of endothelial progenitor cells from adipose tissue as potential angiogenic cell sources for bladder angiogenesis. PLoS ONE 2015, 10, e0117644. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, L.; Xu, Z.; Xu, L.; Xu, Z.; Ping, W.; Liu, J.; Zhou, C.; Wang, M.; Jia, P. Hypoxia-preconditioned adipose-derived endothelial progenitor cells promote bladder augmentation. Tissue Eng. Part A 2020, 26, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Salybekov, A.A.; Kobayashi, S.; Asahara, T. Characterization of endothelial progenitor cell: Past, present, and future. Int. J. Mol. Sci. 2022, 23, 7697. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.H.; Miraglia, S.; Zanjani, E.D.; Almeida-Porada, G.; Ogawa, M.; Leary, A.G.; Olweus, J.; Kearney, J.; Buck, D.W. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997, 90, 5002–5012. [Google Scholar] [CrossRef]
- Peichev, M.; Naiyer, A.J.; Pereira, D.; Zhu, Z.; Lane, W.J.; Williams, M.; Oz, M.C.; Hicklin, D.J.; Witte, L.; Witte, M.A.S.; et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood 2000, 95, 952–958. [Google Scholar] [CrossRef]
- Gehling, U.M.; Ergün, S.; Schumacher, U.; Wagener, C.; Pantel, K.; Otte, M.; Schuch, G.; Schafhausen, P.; Mende, T.; Kilic, N.; et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 2000, 95, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Janic, B.; Guo, A.M.; Iskander, A.S.M.; Varma, N.R.S.; Scicli, A.G.; Arbab, A.S. Human cord blood-derived AC133+ progenitor cells preserve endothelial progenitor characteristics after long term in vitro expansion. PLoS ONE 2010, 5, e9173. [Google Scholar] [CrossRef] [PubMed]
- Bachelier, K.; Bergholz, C.; Friedrich, E.B. Differentiation potential and functional properties of a CD34-CD133+ subpopulation of endothelial progenitor cells. Mol. Med. Rep. 2020, 21, 501–507. [Google Scholar] [CrossRef]
- Friedrich, E.B.; Walenta, K.; Scharlau, J.; Nickenig, G.; Werner, N. CD34−/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ. Res. 2006, 98, e20–e25. [Google Scholar] [CrossRef]
- Cañizo, M.C.; Lozano, F.; González-Porras, J.R.; Barros, M.; López-Holgado, N.; Briz, E.; Sánchez-Guijo, F.M. Peripheral endothelial progenitor cells (CD133+) for therapeutic vasculogenesis in a patient with critical limb ischemia. One year follow-up. Cytotherapy 2007, 9, 99–102. [Google Scholar] [CrossRef]
- Arici, V.; Perotti, C.; Fabrizio, C.; Del Fante, C.; Ragni, F.; Alessandrino, F.; Viarengo, G.; Pagani, M.; Moia, A.; Tinelli, C.; et al. Autologous immuno magnetically selected CD133+ stem cells in the treatment of no-option critical limb ischemia: Clinical and contrast enhanced ultrasound assessed results in eight patients. J. Transl. Med. 2015, 13, 342. [Google Scholar] [CrossRef]
- Patschan, D.; Kribben, A.; Müller, G.A. Postischemic microvasculopathy and endothelial progenitor cell-based therapy in ischemic AKI: Update and perspectives. Am. J. Physiol. Renal. Physiol. 2016, 311, F382–F394. [Google Scholar] [CrossRef]
- Zhou, Z.; Shen, Z.; Chen, X. Tale of Two Magnets: An Advanced Magnetic Targeting System. ACS Nano 2020, 14, 7–11. [Google Scholar] [CrossRef]
- Liu, M.; Yu, W.; Zhang, F.; Liu, T.; Li, K.; Lin, M.; Wang, Y.; Zhao, J.; Jiang, J. Fe3O4@ Polydopamine-Labeled MSCs Targeting the Spinal Cord to Treat Neuropathic Pain Under the Guidance of a Magnetic Field. Int. J. Nanomed. 2021, 16, 3275–3292. [Google Scholar] [CrossRef]
- Ottersbach, A.; Mykhaylyk, O.; Heidsieck, A.; Eberbeck, D.; Rieck, S.; Zimmermann, K.; Breitbach, M.; Engelbrecht, B.; Brügmann, T.; Hesse, M.; et al. Improved heart repair upon myocardial infarction: Combination of magnetic nanoparticles and tailored magnets strongly increases engraftment of myocytes. Biomaterials 2018, 155, 176–190. [Google Scholar] [CrossRef]
- Cores, J.; Caranasos, T.G.; Cheng, K. Magnetically targeted stem cell delivery for regenerative medicine. J. Funct. Biomater. 2015, 6, 526–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Yang, R.; Rahman, M.; Sequeira, R.C.; Cao, N.; Zhang, Y.; Zhao, W.; Fu, Q. Magnetic targeting of super-paramagnetic iron oxide nanoparticle labeled myogenic-induced adipose-derived stem cells in a rat model of stress urinary incontinence. Nanomedicine 2020, 30, 102281. [Google Scholar] [CrossRef]
- Ma, P.a.; Xiao, H.; Yu, C.; Liu, J.; Cheng, Z.; Song, H.; Zhang, X.; Li, C.; Wang, J.; Gu, Z.; et al. Enhanced cisplatin chemotherapy by iron oxide nanocarrier-mediated generation of highly toxic reactive oxygen species. Nano Lett. 2017, 17, 928–937. [Google Scholar] [CrossRef]
- Verma, J.; Lal, S.; Van Noorden, C.J.F. Nanoparticles for hyperthermic therapy: Synthesis strategies and applications in glioblastoma. Int. J. Nanomed. 2014, 9, 2863–2877. [Google Scholar]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Chen, L.; Xie, J.; Wu, H.; Zang, F.; Ma, M.; Hua, Z.; Gu, N.; Zhang, Y. Improving sensitivity of magnetic resonance imaging by using a dual-targeted magnetic iron oxide nanoprobe. Colloids Surf. B Biointerfaces 2018, 161, 339–346. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Wu, H.; Li, J.; Xie, J.; Zang, F.; Ma, M.; Gu, N.; Zhang, Y. Magnetic targeting combined with active targeting of dual-ligand iron oxide nanoprobes to promote the penetration depth in tumors for effective magnetic resonance imaging and hyperthermia. Acta Biomater. 2019, 96, 491–504. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, L.; Shen, J.; Song, Q.; Wu, R.; Ge, Y.; Xin, H.; Zhu, J.; Wu, J.; Jia, P.; et al. Preischemic administration of nonexpanded adipose stromal vascular fraction attenuates acute renal ischemia/reperfusion injury and fibrosis. Stem Cells Transl. Med. 2016, 5, 1277–1288. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, L.; Liu, J.; Xu, L.; Xu, Z.; Chen, Z.; Ge, Y.; Zhao, F.; Wu, R.; Wang, X.; et al. Kidney extracellular matrix hydrogel enhances therapeutic potential of adipose-derived mesenchymal stem cells for renal ischemia reperfusion injury. Acta Biomater. 2020, 115, 250–263. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, M.; Ogo, S.; Ikemoto, M.; Totsuka, Y.; Ishino, K.; Wakabayashi, K.; Yagi, T. Genotoxicity and reactive oxygen species production induced by magnetite nanoparticles in mammalian cells. J. Toxicol. Sci. 2013, 38, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cohen, A.; Hudson, T.E.; Motlagh, D.; Amrani, D.L.; Duffield, J.S. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation 2010, 121, 2211–2220. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Zhang, B.F.; Jiang, H.; Chen, J.; Hu, Q.; Yang, S.; Liu, X.P. Silica-coated magnetic nanoparticles labeled endothelial progenitor cells alleviate ischemic myocardial injury and improve long-term cardiac function with magnetic field guidance in rats with myocardial infarction. J. Cell Physiol. 2019, 234, 18544–18559. [Google Scholar] [CrossRef]
- Patschan, D.; Hildebrandt, A.; Rinneburger, J.; Wessels, J.T.; Patschan, S.; Becker, J.U.; Henze, E.; Krüger, H.A. The hormone melatonin stimulates renoprotective effects of “early outgrowth” endothelial progenitor cells in acute ischemic kidney injury. Am. J. Physiol. Renal. Physiol. 2012, 302, F1305–F1312. [Google Scholar] [CrossRef]
- Patschan, D.; Patschan, S.; Wessels, J.T.; Becker, J.U.; David, S.; Henze, E.; Goligorsky, M.S.; Müller, G.A. Epac-1 activator 8-O-cAMP augments renoprotective effects of allogeneic murine EPCs in acute ischemic kidney injury. Am. J. Physiol. Renal. Physiol. 2010, 298, F78–F85. [Google Scholar] [CrossRef]
- Patschan, D.; Rinneburger, J.; Idrizi, N.; Backhaus, R.; Schwarze, K.; Henze, E.; Patschan, S.; Müller, G.A. Angiopoietin-1 treated early endothelial outgrowth cells (eEOCs) are activated in vitro and reduce renal damage in murine acute ischemic kidney injury (iAKI). BMC Nephrol. 2013, 14, 227. [Google Scholar] [CrossRef]
- Wei, H.; Hu, Y.; Wang, J.; Gao, X.; Qian, X.; Tang, M. Superparamagnetic iron oxide nanoparticles: Cytotoxicity, metabolism, and cellular behavior in biomedicine applications. Int. J. Nanomed. 2021, 16, 6097–6113. [Google Scholar] [CrossRef]
- Pongrac, I.M.; Pavičić, I.; Milić, M.; Brkić Ahmed, L.; Babič, M.; Horák, D.; Vinković Vrček, I. Oxidative stress response in neural stem cells exposed to different superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2016, 11, 1701–1715. [Google Scholar]
- Li, Q.; Zhang, X.; Peng, Y.; Chai, H.; Xu, Y.; Wei, J.; Ren, X.; Wang, X.; Liu, W.; Chen, M.; et al. Comparison of the sorting efficiency and influence on cell function between the sterile flow cytometry and immunomagnetic bead purification methods. Prep. Biochem. Biotechnol. 2013, 43, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Barzegar Behrooz, A.; Syahir, A.; Ahmad, S. CD133: Beyond a cancer stem cell biomarker. J. Drug Target 2019, 27, 257–269. [Google Scholar] [CrossRef]
- Duan, Y.; Yu, S.; Xu, P.; Wang, X.; Feng, X.; Mao, Z.; Gao, C. Co-immobilization of CD133 antibodies, vascular endothelial growth factors, and REDV peptide promotes capture, proliferation, and differentiation of endothelial progenitor cells. Acta Biomater. 2019, 96, 137–148. [Google Scholar] [CrossRef]
- Priante, G.; Gianesello, L.; Ceol, M.; Del Prete, D.; Anglani, F. Cell death in the kidney. Int. J. Mol. Sci. 2019, 20, 3598. [Google Scholar] [CrossRef]
- He, Z.; Wang, H.; Yue, L. Endothelial progenitor cells-secreted extracellular vesicles containing microRNA-93-5p confer protection against sepsis-induced acute kidney injury via the KDM6B/H3K27me3/TNF-α axis. Exp. Cell Res. 2020, 395, 112173. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Liu, W.; Liu, S.; Wang, X.Y.; Diao, Z.L.; Zhang, A.H.; Guo, W.; Han, X.; Dong, X.; et al. Endothelial progenitor cells-derived exosomal microRNA-21-5p alleviates sepsis-induced acute kidney injury by inhibiting RUNX1 expression. Cell Death Dis. 2021, 12, 335. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Liu, J.; Zhou, C.; Ma, W.; Zhou, L.; Ge, Y.; Jia, R. Immunomagnetic Delivery of Adipose-Derived Endothelial Progenitor Cells for the Repair of Renal Ischemia–Reperfusion Injury in a Rat Model. Bioengineering 2023, 10, 509. https://doi.org/10.3390/bioengineering10050509
Wu D, Liu J, Zhou C, Ma W, Zhou L, Ge Y, Jia R. Immunomagnetic Delivery of Adipose-Derived Endothelial Progenitor Cells for the Repair of Renal Ischemia–Reperfusion Injury in a Rat Model. Bioengineering. 2023; 10(5):509. https://doi.org/10.3390/bioengineering10050509
Chicago/Turabian StyleWu, Di, Jingyu Liu, Changcheng Zhou, Wenjie Ma, Liuhua Zhou, Yuzheng Ge, and Ruipeng Jia. 2023. "Immunomagnetic Delivery of Adipose-Derived Endothelial Progenitor Cells for the Repair of Renal Ischemia–Reperfusion Injury in a Rat Model" Bioengineering 10, no. 5: 509. https://doi.org/10.3390/bioengineering10050509