Green Synthesis and Antiproliferative Activity of Gold Nanoparticles of a Controlled Size and Shape Obtained Using Shock Wave Extracts from Amphipterygium adstringens
Abstract
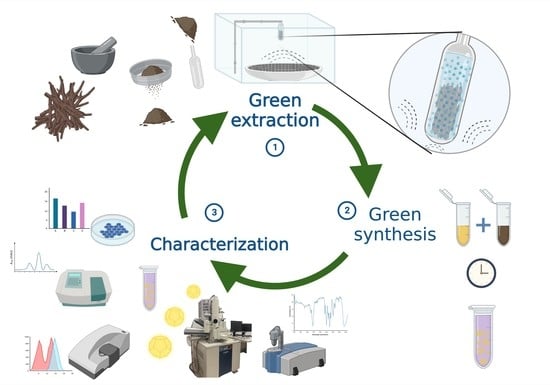
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining Green Extracts
2.3. Optimization of Parameters for Green Extraction Methods
2.4. Antioxidant Capacity
2.5. Total Phenol and Flavonoid Content
2.6. Green Synthesis of AuNPs
2.7. AuNP Characterization
2.8. Viability Assay in Leukemia Cell Lines
2.8.1. Cell Culture
2.8.2. Cytotoxicity Assay with Trypan Blue
2.8.3. Statistics
3. Results and Discussion
3.1. Antioxidant Capacity
3.2. Green Synthesis of AuNPs
3.3. AuNP Characterization
3.4. Biological Assays
Viability Assay in Leukemia Cells Treated with AuNPs (Au@SWCD-EW and Au@US-W), SWCD-EW, and US-W Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez, A. Herbolaria Mexicana, 2nd ed.; Biblioteca Básica de Agricultura: Guadalajara, Jalisco, 2020. [Google Scholar]
- Gnanaprakasam, J.N.R.; Estrada-Muñiz, E.; Vega, L. The antineoplastic agent anacardic 6-pentadecyl salicylic acid produces immunomodulation in vivo via the activation of MAPKs. Toxicol. Appl. Pharm. Pharmacol. 2019, 376, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, X.; Cai, H.; Zhang, P.; Kong, D.; Ge, X.; Du, M.; Liang, R.; Dong, W. Anticancer effects of plant derived anacardic acid on human breast cancer MDA-MB-231 cells. Am. J. Transl. Res. 2018, 10, 2424–2434. [Google Scholar]
- Enea, M.; Pereira, E.; Costa, J.; Soares, M.E.; da Silva, D.D.; de Lourdes Bastos, M.; Carmo, H.F. Cellular uptake and toxicity of gold nanoparticles on two distinct hepatic cell models. Toxicol. Vitr. 2021, 70, 105046. [Google Scholar] [CrossRef] [PubMed]
- Lasagna-Reeves, C.; Gonzalez-Romero, D.; Barria, M.A.; Olmedo, I.; Clos, A.; Ramanujam, V.S.; Urayama, A.; Vergara, L.; Kogan, M.J.; Soto, C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2017, 393, 649–655. [Google Scholar] [CrossRef]
- Anastas, T.; Williamson, P. Green Chemistry: Designing Chemistry for the Environment; American Chemical Society: Washington, DC, USA, 1994; Volume 12. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Pal, G.; Rai, P.; Pandey, A. Green synthesis of nanoparticles: A greener approach for a cleaner future. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–26. [Google Scholar] [CrossRef]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E Low. Dimens. Syst. Nanostruct. 2010, 42, 1417–1424. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Synthesis of Gold Nanoparticles using Plant Extract: An Overview. Nano Res. Appl. 2015, 1, 1. Available online: http://www.imedpub.com2015http://nanotechnology.imedpub.com/archive.php (accessed on 1 January 2020).
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC–Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Molina, G.A.; González-Fuentes, F.; Loske, A.M.; Fernández, F.; Estevez, M. Shock wave-assisted extraction of phenolic acids and flavonoids from Eysenhardtia polystachya heartwood: A novel method and its comparison with conventional methodologies. Ultrason. Sonochem 2020, 61, 104809. [Google Scholar] [CrossRef]
- Rassweiler, J.; Bergsdorf, T.; Bohris, C.; Burkhardt, M.; Burnes, L.; Forssmann, B. Consensus: Shock. Wave Technology and Application-State of the Art in 2010; Georg ThiemeVerlag: Sttutgart, NY, USA, 2010. [Google Scholar]
- Magaña-Ortíz, D.; Coconi-Linares, N.; Ortiz-Vazquez, E.; Fernández, F.; Loske, A.M.; Gómez-Lim, M.A. A novel and highly efficient method for genetic transformation of fungi employing shock waves. Fungal Genet. Biol. 2013, 56, 9–16. [Google Scholar] [CrossRef]
- Lohrer, H.; Gerdesmeyer, L. (Eds.) Shock wave therapy in practice. In Multidisciplinary Medical Applications; Bruchverlag: Heilbronn, Germany, 2014. [Google Scholar]
- Loske, A.M.; Fernández, F.; Magaña-Ortíz, D.; Coconi-Linares, N.; Ortíz-Vázquez, E.; Gómez-Lim, M.A. Tandem shock waves to enhance genetic transformation of Aspergillus niger. Shock. Waves 2014, 26, 1–23. [Google Scholar] [CrossRef]
- Millán-Chiu, B.; Camacho, G.; Varela-Echavarría, A.; Tamariz, E.; Fernández, F.; López-Marín, L.M.; Loske, A.M. Shock waves and DNA-cationic lipid assemblies: A synergistic approach to express exogenous genes in human cells. Ultrasoun Med. Biol. 2014, 40, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.L.; Magaña-Ortíz, D.; Gómez-Lim, M.; Fernández, F.; Loske, A.M. Physical methods for genetic transformation of fungi and yeast. Phys. Life Rev. 2014, 11, 184–203. [Google Scholar] [CrossRef]
- Lopez-Marin, L.M.; Millán-Chiu, B.E.; Castaño-González, K.; Aceves, C.; Fernández, F.; Varela-Echavarría, A.; Loske, A.M. Shock Wave-Induced Damage and Poration in Eukaryotic Cell Membranes. J. Membr. Biol. 2017, 250, 41–52. [Google Scholar] [CrossRef]
- Loske, A.M. Medical and Biomedical Applications of Shock Waves; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Wang, C.; Schaden, W.; Ko, J. Shockwave Medicine in: Translational Research in Biomedicine; Karger Medical and Scientific Publishers: Basel, Switerland, 2018. [Google Scholar]
- Peña, M.; Delgado-Gonzalez, E.; López-Marín, L.M.; Millán-Chiu, B.E.; Fernández, F.; Rodriguez-Castelan, J.; Muñoz-Torres, C.; Carrasco, G.; Anguiano, B.; Loske, A.M.; et al. Shock wave application increases the antineoplastic effect of molecular iodine supplement in breast cancer xenografts. Ultrasound Med. Biol. 2020, 46, 640–659. [Google Scholar] [CrossRef] [PubMed]
- Philipp, A.; Delius, M.; Scheffczyk, C.; Vogel, A.; Lauterborn, W. Interaction of litrotropher-generated shock waves with air bubbles. J. Acoust. Soc. Am. 1993, 93, 2496–2509. [Google Scholar] [CrossRef]
- Kodama, T.; Takayama, K. Dynamic behavior of bubbles during extracorporeal shock-wave lithotripsy. Ultrasound Med. Biol. 1998, 24, 723–738. [Google Scholar] [CrossRef]
- Ohl, C.; Ikink, R. Shock-wave-induced jetting of micron-size bubbles. Phys. Rev. Lett. 2003, 9, 214502. [Google Scholar] [CrossRef] [Green Version]
- Klaseboer, E.; Fong, S.W.; Turangan, C.K.; Khoo, B.C.; Szeri, A.J.; Calvisi, M.L.; Sankin, G.N.; Zhong, P. Interaction of litrotripter shockwaves with single inertial cavitation bubbles. J. Fluid. Mech. 2007, 593, 33–56. [Google Scholar] [CrossRef]
- Sapozhnikov, O.; Maxwell, A.; MacConaghy, B.; Bailey, M.R. A mechanistic analysis of stone fracture in lithotripsy. J. Acoust. S. Am. 2007, 121, 1190–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brujan, E.A.; Ikeda, T.; Matsumoto, Y. On the pressure of cavitation bubbles. Exp. Therm. Fluid. Sci. 2008, 32, 1188–1191. [Google Scholar] [CrossRef]
- Johnsen, E.; Colonius, T. Shock-induced collapse of a gas bubble in shock wave lithotripsy. J. Acoust. Soc. Am. 2008, 124, 2011–2020. [Google Scholar] [CrossRef]
- Brujan, E.; Ikeda, T.; Yoshinaka, K.; Matsumoto, Y. The final stage of the collapse of a cloud of bubbles close to a rigid boundary. Sonochemistry 2011, 18, 59–64. [Google Scholar] [CrossRef]
- Ohl, S.W.; Klaseboer, E.; Khoo, B.C. Bubbles with shock waves and ultrasound: A review. Interface Focus 2015, 5, 20150019. [Google Scholar] [CrossRef]
- Tzanakis, I.; Eskin, D.G.; Georgoulas, A.; Fytanidis, D.K. Incubation pit analysis and calculation of the hydrodynamic impact pressure from the implosion of an acoustic cavitation bubble. Ultrason. Sonochem. 2014, 21, 866–878. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Zill-E-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Liu, N.; Wang, K.; Gao, Y.; Li, D.; Lin, W.; Li, C. Tartrate as a substitute of citrate to prepare gold colloids from chloroauric acid. Colloids Surf. A Phys. Physicochem. Eng. Asp. 2017, 535, 251–256. [Google Scholar] [CrossRef]
- Devi, C.; Boro, B.; Barthakur, M.; Reddy, P.V.B.; Kalita, P. Effect of citrate stabilized gold nanoparticle on the biochemical and histological alterations of liver in an experimental animal model. Mater. Today Proc. 2020, 46, 6409–6414. [Google Scholar] [CrossRef]
- Sun, P.P.; Lai, C.S.; Hung, C.J.; Dhaiveegan, P.; Tsai, M.L.; Chiu, C.L.; Fang, J.M. Subchronic oral toxicity evaluation of gold nanoparticles in male and female mice. Heliyon 2021, 7, e06577. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.; Vian, M.; Cavalluzzi, M.M.; Franchini, C.; Corbo, F.; Lentini, G.; Chemat, F. Ultrasound and deep eutectic solvents: An efficient combination to tune the mechanism of steviol glycosides extraction. Ultrason. Sonochem. 2020, 69, 105255. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Prieto, Á.; de Icaza-Herrera, M.; Loske, A.M.; Castaño-Tostado, E. Kriging model to study the dynamics of a bubble subjected to tandem shock waves as used in biomedical applications. Ultrasonics 2019, 91, 10–18. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Aguilar-Villalva, R.; Molina, G.A.; España-Sánchez, B.L.; Díaz-Peña, L.F.; Elizalde-Mata, A.; Valerio, E.; Azanza-Ricardo, C.; Estevez, M. Antioxidant capacity and antibacterial activity from Annona cherimola phytochemicals by ultrasound-assisted extraction and its comparison to conventional methods. Arab. J. Chem. 2021, 14, 103239. [Google Scholar] [CrossRef]
- Souza, M.C.; Santos, M.P.; Sumere, B.R.; Silva, L.C.; Cunha, D.T.; Martinez, J.; Barbero, G.F.; Rostagno, M.A. Isolation of gallic acid, caffeine and flavonols from black tea by on-line coupling of pressurized liquid extraction with an adsorbent for the production of functional bakery products. Lwt 2020, 117, 108661. [Google Scholar] [CrossRef]
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod. Process. 2020, 122, 136–144. [Google Scholar] [CrossRef]
- Hurtado, R.B.; Cortez-Valadez, M.; Ramírez-Rodríguez, L.P.; Larios-Rodriguez, E.; Alvarez, R.A.; Rocha-Rocha, O.; Delgado-Beleño, Y.; Martinez-Nuñez, C.E.; Arizpe-Chávez, H.; Hernández-Martínez, A.R.; et al. Instant synthesis of gold nanoparticles at room temperature and SERS applications. Phys. Lett. Sect. A Gen. General. At. Solid. State Phys. 2016, 380, 2658–2663. [Google Scholar] [CrossRef]
- Vergara-Castañeda, H.; Granados-Segura, L.O.; Luna-Bárcenas, G.; McClements, D.J.; Herrera-Hernández, M.G.; Arjona, N.; Hernández-Martínez, A.R.; Estevez, M.; Pool, H. Gold nanoparticles bioreduced by natural extracts of arantho (Kalanchoe daigremontiana) for biological purposes: Physicochemical, antioxidant and antiproliferative evaluations. Mater. Res. Express 2019, 6, 055010. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Chmelová, D.; Škulcová, D.; Legerská, B.; Horník, M.; Ondrejovič, M. Ultrasonic-assisted extraction of polyphenols and antioxidants from Picea abies bark. J. Biotechnol. 2020, 314–315, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Gold and silver nanoparticles: A class of chromophores with colors tunable in the range from 400 to 750 nm. Analyst 2003, 128, 686–691. [Google Scholar] [CrossRef]
- Jeon, H.B.; Tsalu, P.V.; Ha, J.W. Shape Effect on the Refractive Index Sensitivity at Localized Surface Plasmon Resonance Inflection Points of Single Gold Nanocubes with Vertices. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehl, C.L.; Hafner, J.H. Shape-dependent plasmon resonances of gold nanoparticles. J. Mater. Chem. 2008, 18, 2415–2419. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Jiang, N.; Zhang, L.; Yin, M. Green synthesis of gold nanoparticles from Fritillaria cirrhosa and its anti-diabetic activity on Streptozotocin induced rats. Arab. J. Chem. 2020, 13, 5096–5106. [Google Scholar] [CrossRef]
- Li, S.; Al-Misned, F.A.; El-Serehy, H.A.; Yang, L. Green synthesis of gold nanoparticles using aqueous extract of Mentha Longifolia leaf and investigation of its anti-human breast carcinoma properties in the in vitro condition. Arab. J. Chem. 2021, 14, 102931. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Almalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti-inflamatory activities. Environ. Chem. Ecotoxicol. 2021, 2, 117–124. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef]
- Sampaio, S.; Viana, J. Production of silver nanoparticles by green synthesis using artichoke (Cynara scolymus L.) aqueous extract and measurement of their electrical conductivity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 045002. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264. [Google Scholar] [CrossRef] [Green Version]
- López-Miranda, J.L.; Esparza, R.; González-Reyna, M.A.; España-Sánchez, B.L.; Hernandez-Martinez, A.R.; Silva, R.; Estévez, M. Sargassum influx on the mexican coast: A source for synthesizing silver nanoparticles with catalytic and antibacterial properties. Appl. Sci. 2021, 11, 4638. [Google Scholar] [CrossRef]
- Silverstein, R.; Webster, F.; Kiemle, D. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons, INC: Hoboken, NJ, USA, 2015. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Cryst. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Zheng, C.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A. Cytotoxicity, anti-acute leukemia, and antioxidant properties of gold nanoparticles green-synthesized using Cannabis sativa L leaf aqueous extract. Arab. J. Chem. 2021, 14, 103060. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Peixoto, I.T.A.; Verde-Star, M.J.; De La Torre-Zavala, S.; Aviles-Arnaut, H.; Ruiz, A.L.T.G. In Vitro Antimicrobial and Antiproliferative Activity of Amphipterygium adstringens. Evid.-Based Complement. Altern. Med. 2015, 2015, 175497. [Google Scholar] [CrossRef] [Green Version]
- Alam-Escamilla, D.; Estrada-Muñiz, E.; Solís-Villegas, E.; Elizondo, G.; Vega, L. Genotoxic and cytostatic effects of 6-pentadecyl salicylic anacardic acid in transformed cell lines and peripheral blood mononuclear cells. Mutat. Res. Genet. Toxicol. Environ. Environ. Mutagen. 2015, 777, 43–53. [Google Scholar] [CrossRef]
- Galot-Linaldi, J.; Hernández-Sánchez, K.M.; Estrada-Muñiz, E.; Vega, L. Anacardic acids from amphipterygium adstringens confer cytoprotection against 5-fluorouracil and carboplatin induced blood cell toxicity while increasing antitumoral activity and survival in an animal model of breast cancer. Molecules 2021, 26, 3241. [Google Scholar] [CrossRef]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. Sci. Rep. 2018, 8, 12295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open. Biol. 2013, 3, 120144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef] [PubMed]












| Sample | Acronym |
|---|---|
| A. adstringens methanolic macerate extraction | Mac-MeOH |
| A. adstringens ethanol:water ultrasound-assisted extraction | US-EW |
| A. adstringens ethanol:water shock wave-assisted extraction constant delay | SWCD-EW |
| A. adstringens ethanol:water shock wave-assisted extraction variable delay | SWVD-EW |
| A. adstringens water ultrasound-assisted extraction | US-W |
| AuNPs synthesized in A. adstringens methanolic macerate extract | Au@Mac-MeOH |
| AuNPs synthesized in A. adstringens ethanol:water ultrasound-assisted extraction | Au@US-EW |
| AuNPs synthesized in A. adstringens water ultrasound-assisted extraction | Au@US-W |
| AuNPs synthesized in A. adstringens ethanol:water shock wave-assisted extraction constant delay | Au@SWCD-EW |
| AuNPs synthesized in A. adstringens ethanol:water shock wave-assisted extraction variable delay | Au@SWVD-EW |
| Sample | TPC (mg/100 mLGAE) a | TFC (mg/100 mL QE) b | DPPH (mM/mL TEAC) c | CUPRAC (mM/mL TEAC) d |
|---|---|---|---|---|
| Mac-MeOH | 30.27 ± 0.25 | 60.06 ± 0.14 | 1359.91 ± 23.38 | 2765 ± 11.05 |
| US-EW | 26.36 ± 0.43 | 75.41 ± 0.32 | 1836.72 ± 10.25 | 3018 ± 13.22 |
| SWCD-EW | 53.31 ± 0.37 | 68.58 ±0.25 | 4838.95 ± 39.23 | 7051 ± 18.24 |
| SWVD-EW | 39.15 ± 0.21 | 90.73 ± 0.18 | 2588.32 ± 50.17 | 5374 ± 36.44 |
| US-W | 57.83 ± 0.32 | 97.96 ± 0.41 | 3537.69 ± 34.81 | 5109 ± 27.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Ortiz, D.; García-Alcocer, G.; Loske, A.M.; Fernández, F.; Becerra-Becerra, E.; Esparza, R.; Gonzalez-Reyna, M.A.; Estevez, M. Green Synthesis and Antiproliferative Activity of Gold Nanoparticles of a Controlled Size and Shape Obtained Using Shock Wave Extracts from Amphipterygium adstringens. Bioengineering 2023, 10, 437. https://doi.org/10.3390/bioengineering10040437
Torres-Ortiz D, García-Alcocer G, Loske AM, Fernández F, Becerra-Becerra E, Esparza R, Gonzalez-Reyna MA, Estevez M. Green Synthesis and Antiproliferative Activity of Gold Nanoparticles of a Controlled Size and Shape Obtained Using Shock Wave Extracts from Amphipterygium adstringens. Bioengineering. 2023; 10(4):437. https://doi.org/10.3390/bioengineering10040437
Chicago/Turabian StyleTorres-Ortiz, Daniela, Guadalupe García-Alcocer, Achim M. Loske, Francisco Fernández, Edgardo Becerra-Becerra, Rodrigo Esparza, Marlen Alexis Gonzalez-Reyna, and Miriam Estevez. 2023. "Green Synthesis and Antiproliferative Activity of Gold Nanoparticles of a Controlled Size and Shape Obtained Using Shock Wave Extracts from Amphipterygium adstringens" Bioengineering 10, no. 4: 437. https://doi.org/10.3390/bioengineering10040437