1. Introduction
The social aging of the population is currently one of the greatest concerns facing nations worldwide [
1]. The number of elderly people with spinal cord injuries, Parkinson’s disease, and other diseases is constantly rising [
2,
3,
4]. The most noticeable symptom of these age-related diseases is tremor, which prevents older people from performing motor activities and stabilizing desired movements [
5]. There are many techniques that have been developed to address this kind of issue, and the most welcomed therapy adopted by medical professionals is functional electrical stimulation (FES) [
6]. FES is an effective therapy for patients who have lost the ability to perform desired motions, which generates body movements artificially by using low-energy electrical pulses [
7]. This therapy was first proposed by Liberson and his co-workers in the year 1961 and aimed to stimulate the peroneal nerve to correct foot drop by triggering a foot switch [
8]. When using FES on patients, different electrical currents of varying number and intensity produce diverse results [
9]. Hence, how to intelligently activate the FES device to provide the electrical pulses has attracted the attention of numerous researchers.
To achieve high-precision, task-specific FES rehabilitation therapy, many researchers started to investigate the mechanism of how the central nervous system (CNS) can control the human body to complete a specific motor task and tried to utilize the FES technique to generate the motion based on this mechanism. For the human arm reaching movement, Bernstein first described this movement in terms of coordinates, which served as the foundation for a qualitative and quantitative analysis of its kinematics and dynamics [
10]. Apart from the investigation from a mathematical aspect, Morraso et al. conducted numerous experiments and the results suggest that human arm movement trajectories are planned in task-oriented coordinates rather than joint coordinates [
11]. Specifically, human arm reaching movement trajectories from the start to the desired position are in the shape of roughly a straight path, and the speed profile of that is in the shape of a bell curve. Subsequently, investigations on the analysis of biomechanical structure, modeling, and development of motor control, etc. for the human arm were sparked. By building a model of the human arm, Uno et al. for the first time analyzed its kinematics and dynamics, providing the theoretical underpinnings for the motor control of arm reaching actions [
12]. Based on these findings, Jagodnik et al. concluded that the feedback gains function in the brain to control joint angles and produce the required movements [
13]. However, because the controller was based on a skeleton arm, none of them gave adequate consideration to the influence of muscles during designing the motor controller. Thomas et al. used an actor–critic architecture in the motor controller to cause the skeletal muscles to contract, causing the arm to move [
14]. To control the movement of a musculoskeletal arm, Tahara et al. used an iterative learning approach [
15]. A fuzzy sliding controller for this musculoskeletal arm was created by Chen et al. to conduct reaching movements [
16]. Hausmann et al. used neural networks to simulate the sensorimotor system of the brain and create smooth trajectories that resembled the actual arm reaching action [
17]. However, all of their designs of motor controllers stray from the fundamentals of neuroscience and are solely intended to drive the arm from one position to another. According to neuroscience studies, the many functional regions of the brain control the arm’s natural movement trajectory [
18]. Therefore, it is crucial to develop a motor controller for FES that is neurophysiology-based, human brain-like, and has multifunctional sections. Apart from the application for FES, this kind of controller can be applied to many other fields such as the energy sector [
19], flight control [
20], vehicle technologies [
21], etc.
To address this issue, in this paper, a neuromechanics-based neural feedback controller for arm reaching movements is developed. Compared to the research mentioned above, the contribution of this paper is threefold. First, the controller is developed based on neuromechanics which deems the motion is generated through the interaction among neural, muscular, and skeletal systems. In detail, this controller transits the neural signals to skeletal muscles, and then the skeletal muscles generate the tensile forces. After that, the skeletons are driven by these tensile forces from the start position towards the target position. This sort of controller operating mechanism is the first one to be employed for arm reaching movements. Second, as the controller is designed based on neuromechanics, a model that mimics the real human arm is needed. Here, a musculoskeletal arm model is constructed, consisting of two skeletons and seven muscles, each of which is modeled after the genuine skeletons and muscles. Third, to prevent the patients from serious muscle strain during arm reaching movements, the controller stimulates the muscles to generate small and steady forces, which are sufficient to drive the arm toward the target position. In other words, with this controller, not too much energy consumption is required to perform arm reaching movements.
The structure of this paper is as follows: In
Section 2, the kinematical and dynamical properties of the human musculoskeletal arm are modeled.
Section 3 describes the mechanism of the neuromechanics-based neural feedback controller.
Section 4 presents the numerical simulation experiments to validate the good performance of the controller.
Section 5 concludes this paper with a summary of the results and discusses the foreseen challenges for the application of neural feedback controllers.
5. Conclusions and Discussion
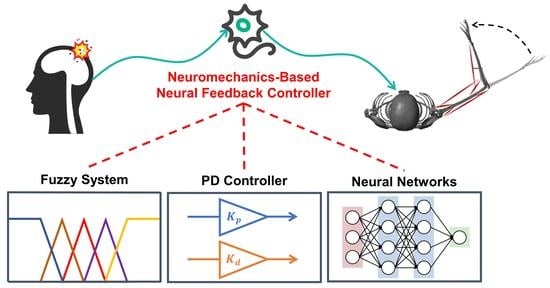
In this paper, a neuromechanics-based neural feedback controller for arm reaching movements is developed. Through conducting the simulation experiments, it is proven to be effective at driving the patient’s arm toward the target position and robust enough to complete different reaching movements tasks. It can also be observed that the controller does not stimulate the skeletal muscle to generate too much tensile force. It means that the patients are protected from experiencing serious muscle strain. To develop a controller with such good performance, first, a musculoskeletal arm model is constructed to simulate the real biomechanical structure of the human arm. Second, based on this model, a neural feedback controller is developed which follows the principles of neuromechanics. In detail, the controller imitates the behavior of the human brain, which consists of three main components: fuzzy system, feedback gains, and neural networks. A fuzzy system is used here to mimic the human brain that cannot determine the precise distance between the current position and the target position but can only approximately compute it. Feedback gains are utilized here since it has already been validated that they change the joint angles and joint angle velocity to fulfil motion tasks. Neural networks are adopted here to learn the actual muscle activity that occurs during the arm reaching movements to ensure that the controller can stimulate the muscles to generate small, steady forces that are sufficient to move the arm from one position to the other. In conclusion, with this neuromechanics-based neural feedback controller, the patient’s arm can be safely driven from the target position to the target position without consuming energy too much due to small and steady muscle forces generated.
A feasible alternative to the approach adopted in this paper, which models the kinematics and dynamics of the skeletal muscle model, analyzes its system characteristics, and then designs a controller, is to use software already available for skeletal muscle systems to train an efficient controller directly through thousands of unrestricted interactions with the musculoskeletal system. A good example of adopting this method is when Wannawas et al. [
36] used neuromechanics-based deep reinforcement learning to control FES during cycling. They first developed a neuromechanical model of cycling based on an open software named “OpenSim”, and then optimized the control strategy via allowing interactions between the musculoskeletal model and the mechanical properties of a bicycle. The results they presented show that their controller can achieve approximately optimal cycling performance. This approach is effective in seeking the near-optimal neural feedback controller. However, because the interactions are based on a very particular model, the performance of the controller is strongly individualized. The trajectories produced by the controller for the upper limb models with different parameter values did not significantly change in the simulation experiments for robustness testing in this paper because only the important parameters of the human upper limb arm are taken into account, providing a tolerance space for system variations.
Although the controller is proven to be effective via numerical simulation experiments, it still has a way to go before it can be used in real life. There are several issues with this gap. First, unlike physical models, which can qualitatively and quantitatively explain a physical occurrence, biological models are unable to do so. The experimental theory of biological simulation cannot be perfectly applied to a real biological scenario since biological models can only provide qualitative explanations for biological phenomena rather than comprehensive quantitative ones. Second, it is challenging to simulate whether the variable amount of delay will have an impact on the control effect because since the nerve signal travels from the brain, stimulates the corresponding muscle, and then produces the corresponding movement, there is frequently a significant time delay between these three events. Third, given that each person’s biological characteristics are unique, even though this paper demonstrates through robustness testing that parameter changes within a specific range will not affect the effectiveness of the controller, when the function of the individual completely changes, the developer must analyze the characteristics of the patient to develop a suitable controller. Therefore, this investigation requires a lot of human and material investment, which is obviously detrimental to the business. Despite the difficulties mentioned above, it cannot be denied that the goal of this paper is to propose a novel, highly effective controller based on neuromechanics, developed specifically for human upper limb planar movements, and to demonstrate a potential solution to the problem of patients who are unable to actively control their movements.