Customised 3D-Printed Surgical Guide for Breast-Conserving Surgery after Neoadjuvant Chemotherapy and Its Clinical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
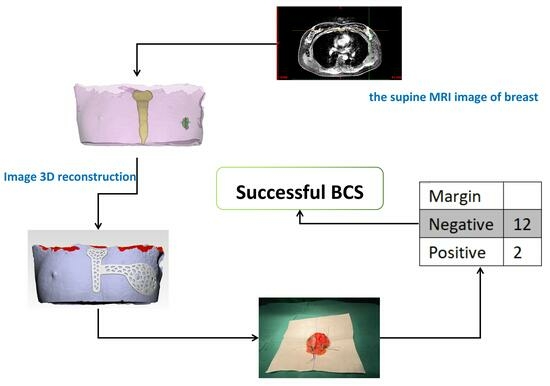
2.2. Design and Manufacturing of the Surgical Guide
2.3. Operation
2.4. Pathological Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- King, T.; Morrow, M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 2015, 12, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Fukada, I.; Araki, K.; Kobayashi, K.; Shibayama, T.; Takahashi, S.; Gomi, N.; Kokubu, Y.; Oikado, K.; Horii, R.; Akiyama, F.; et al. Pattern of Tumor Shrinkage during Neoadjuvant Chemotherapy Is Associated with Prognosis in Low-Grade Luminal Early Breast Cancer. Radiology 2018, 286, 49–57. [Google Scholar] [CrossRef]
- Yamashiro, N.; Tozaki, M.; Ogawa, T.; Kawano, N.; Suzuki, T.; Ozaki, S.; Sakamoto, N.; Abe, S.; Fukuma, E. Preoperative MRI marking technique for the planning of breast-conserving surgery. Breast Cancer 2009, 16, 223–228. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, W.; Zhang, X.; He, S.; Shao, N.; Shi, H.; Lin, Z.; Wu, X.; Li, T.; Lin, H.; et al. Prediction of Tumor Shrinkage Pattern to Neoadjuvant Chemotherapy Using a Multiparametric MRI-Based Machine Learning Model in Patients with Breast Cancer. Front. Bioeng. Biotechnol. 2021, 9, 662749. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, H.H.; Ahn, J.H.; Kim, S.B.; Jung, K.H.; Gong, G.; Son, B.H.; Ahn, S.H. Comparison of mammography, sonography, MRI and clinical examination in patients with locally advanced or inflammatory breast cancer who underwent neoadjuvant chemotherapy. Br. J. Radiol. 2011, 84, 612–620. [Google Scholar] [CrossRef]
- Londero, V.; Bazzocchi, M.; Del Frate, C.; Puglisi, F.; Di Loreto, C.; Francescutti, G.; Zuiani, C. Locally advanced breast cancer: Comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur. Radiol. 2004, 14, 1371–1379. [Google Scholar] [CrossRef]
- Kopans, D.B.; Swann, C.A. Preoperative imaging-guided needle placement and localization of clinically occult breast lesions. AJR Am. J. Roentgenol. 1989, 152, 1–9. [Google Scholar] [CrossRef]
- Canavese, G.; Catturich, A.; Vecchio, C.; Tomei, D.; Estienne, M.; Moresco, L.; Imperiale, A.; Parodi, G.C.; Massa, T.; Badellino, F. Pre-operative localization of non-palpable lesions in breast cancer by charcoal suspension. Eur. J. Surg. Oncol. 1995, 21, 47–49. [Google Scholar] [CrossRef]
- Thomassin-Naggara, I.; Lalonde, L.; David, J.; Darai, E.; Uzan, S.; Trop, I. A plea for the biopsy marker: How, why and why not clipping after breast biopsy? Breast Cancer Res. Treat. 2012, 132, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Galimberti, V.; Trifiro, G.; De Cicco, C.; Peradze, N.; Brenelli, F.; Fernandez-Rodriguez, J.; Rotmensz, N.; Latronico, A.; Berrettini, A.; et al. Occult breast lesion localization plus sentinel node biopsy (SNOLL): Experience with 959 patients at the European Institute of Oncology. Ann. Surg. Oncol. 2007, 14, 2928–2931. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.K. Update on Preoperative Breast Localization. Radiol. Clin. N. Am. 2017, 55, 591–603. [Google Scholar] [CrossRef]
- Somasundaram, S.K.; Potter, S.; Elgammal, S.; Maxwell, A.J.; Sami, A.S.; Down, S.K.; Dave, R.V.; Harvey, J. Impalpable breast lesion localisation, a logistical challenge: Results of the UK iBRA-NET national practice questionnaire. Breast Cancer Res. Treat. 2021, 185, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Corsi, F.; Sorrentino, L.; Bossi, D.; Sartani, A.; Foschi, D. Preoperative localization and surgical margins in conservative breast surgery. Int. J. Surg. Oncol. 2013, 2013, 793819. [Google Scholar] [CrossRef]
- Ning, H.; Wang, X.; Shi, L. Photoinhibiting via simultaneous photoabsorption and free-radical reaction for high-fidelity light-based bioprinting. Nat. Commun. 2023, 14, 3063. [Google Scholar]
- Zhu, X.; Chen, F.; Cao, H.; Li, L.; He, N.; Han, X. Design and fused deposition modeling of triply periodic minimal surface scaffolds with channels and hydrogel for breast reconstruction. Int. J. Bioprint. 2023, 9, 685. [Google Scholar] [CrossRef]
- Krieger, K.J.; Bertollo, N.; Dangol, M. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 42. [Google Scholar] [CrossRef]
- Barth, R.J., Jr.; Krishnaswamy, V.; Paulsen, K.D.; Rooney, T.B.; Wells, W.A.; Rizzo, E.; Angeles, C.V.; Marotti, J.D.; Zuurbier, R.A.; Black, C.C. A Patient-Specific 3D-Printed Form Accurately Transfers Supine MRI-Derived Tumor Localization Information to Guide Breast-Conserving Surgery. Ann. Surg. Oncol. 2017, 24, 2950–2956. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Kim, G.B.; Choi, S.; Lee, S.; Kim, N.; Ko, B. Breast-Conserving Surgery after Neoadjuvant Chemotherapy Using a Three-Dimensional-Printed Surgical Guide Based on Supine Magnetic Resonance Imaging: A Case Report. J. Breast Cancer 2021, 24, 235–240. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Kim, H.J.; Lee, J.; Chung, I.Y.; Kim, J.; Lee, S.; Son, B.H.; Ahn, S.H.; Kim, H.H.; Seo, J.B.; et al. Breast-conserving surgery with 3D-printed surgical guide: A single-center, prospective clinical study. Sci. Rep. 2021, 11, 2252. [Google Scholar] [CrossRef] [PubMed]
- Cappello, I.A.; Candelari, M.; Pannone, L.; Monaco, C.; Bori, E.; Talevi, G.; Ramak, R.; La Meir, M.; Gharaviri, A.; Chierchia, G.B.; et al. 3D Printed Surgical Guide for Coronary Artery Bypass Graft: Workflow from Computed Tomography to Prototype. Bioengineering 2022, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Postl, L.; Mücke, T.; Hunger, S.; Bissinger, O.; Malek, M.; Holberg, S.; Burgkart, R.; Krennmair, S. In-house 3D-printed surgical guides for osseous lesions of the lower jaw: An experimental study. Eur. J. Med. Res. 2021, 26, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Yang, X.; Fan, L.; Qi, X.; Chen, Q.; Jiang, J. Shrink pattern of breast cancer after neoadjuvant chemotherapy and its correlation with clinical pathological factors. World J. Surg. Onc. 2013, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Burstein, H.J.; Winter, E.P.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.-J.; et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol. 2019, 30, 1181. [Google Scholar] [CrossRef]
- Goorts, B.; van Nijnatten, T.J.; de Munck, L.; Moossdorff, M.; Heuts, E.M.; de Boer, M.; Lobbes, M.B.; Smidt, M.L. Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res. Treat. 2017, 163, 83–91. [Google Scholar] [CrossRef]
- Clarke, M.; Coates, A.S.; Darby, S.C.; Davies, C.; Gelber, R.D.; Godwin, J.; Goldhirsch, A.; Gray, R.; Peto, R.; Pritchard, K.I.; et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: Patient-level meta-analysis of randomised trials. Lancet 2008, 371, 29–40. [Google Scholar]
- Wolmark, N.; Wang, J.; Mamounas, E.; Bryant, J.; Fisher, B. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project, B-18. J. Natl. Cancer Inst. Monogr. 2001, 30, 96–102. [Google Scholar] [CrossRef]
- Partridge, S.C.; Gibbs, J.E.; Lu, Y.; Esserman, L.J.; Sudilovsky, D.; Hylton, N.M. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am. J. Roentgenol. 2002, 179, 1193–1199. [Google Scholar] [CrossRef]
- Goorts, B.; Dreuning, K.M.A.; Houwers, J.B.; Kooreman, L.F.S.; Boerma, E.G.; Mann, R.M.; Lobbes, M.B.I.; Smidt, M.L. MRI-based response patterns during neoadjuvant chemotherapy can predict pathological (complete) response in patients with breast cancer. Breast Cancer Res. 2018, 20, 34. [Google Scholar] [CrossRef]
- Dixon, J.M.; Houssami, N. Bigger margins are not better in breast conserving surgery. Br. Med. J. 2012, 345, e5855. [Google Scholar] [CrossRef] [PubMed]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.-U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. CARS 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Marro, A.; Bandukwala, T.; Mak, M. Three-Dimensional Printing and Medical Imaging: A Review of the Methods and Applications. Curr. Probl. Diagn. Radiol. 2016, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Siddique, N.; Paheding, S.; Elkin, C.P.; Devabhaktuni, V. U-Net and Its Variants for Medical Image Segmentation: A Review of Theory and Applications. IEEE Access 2021, 9, 82031–82057. [Google Scholar] [CrossRef]






| Variables | N (%) | |
|---|---|---|
| Age (years) | Median | 48 |
| Range | 26–68 | |
| <50 | 12 (60.0) | |
| >50 | 8 (40.0) | |
| Pathology | Invasive ductal carcinoma | 18 (90.0) |
| Invasive lobular carcinoma | 2 (10.0) | |
| Stage | cT2N0M0 | 8 (40.0) |
| cT2N1M0 | 8 (40.0) | |
| cT2N2M0 | 1 (5.0) | |
| cT2N3M0 | 2 (10.0) | |
| cT2N3M1 | 1 (5.0) | |
| Subtype | HR+/HER2+ | 3 (15.0) |
| HR-/HER2+ | 8 (40.0) | |
| Luminal A | 1 (5.0) | |
| Luminal B | 2 (10.0) | |
| TN | 6 (30.0) | |
| Histologic grade | II | 12 (60.0) |
| III | 8 (40.0) | |
| Lymph node status | Positive | 4 (20.0) |
| Negative | 16 (80.0) | |
| Patient ID | Pathology (IIC or IDC) | Stage | IHC | Subtype | Histologic Grade | Lymph Node Status | Neoadjuvant Therapy | Margin Status | pCR | Surgery Type |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IIC | cT2N0M0 | ER (−), PR (−), HER-2(3+), Ki67(10%) | HR-/HER2+ | II | Negative | TCbHP X6 | Negative | Non-pCR | BSG |
| 2 | IDC | cT2N0M0 | ER(3+ 90%), PR(2+ 70%), HER-2(1+), Ki67(20%) | Luminal A | II | Negative | AC-T X8 | Negative | Non-pCR | BSG |
| 3 | IDC | cT2N2M0 | ER(3+ 30%), PR(−), HER-2(3+), Ki67(50%) | HR+/HER2+ | III | Negative | THP X5 | Negative | Non-pCR | BSG |
| 4 | IDC | cT2N0M0 | ER(−), PR(−), Her2(2+), Ki67(40%+) | HR-/HER2+ | III | Negative | TCbH X6 | Negative | Non-pCR | Other |
| 5 | IIC | cT2N1M0 | ER(2+ 80%), PR(3+ 80%), Her2(3+), Ki67(40%) | HR+/HER2+ | II | Negative | AC-THP X8 | Positive | Non-pCR | BSG |
| 6 | IDC | cT2N0M0 | ER(−), PR(2+ 50%), Her2(0), Ki67(10%) | Luminal B | II | Negative | TAC X6 | Negative | Non-pCR | BSG |
| 7 | IDC | cT2N0M0 | ER(−), PR(−), Her2(0), Ki67(35%) | TN | III | Negative | TAC X6 | Negative | pCR | Other |
| 8 | IDC | cT2N0M0 | ER(−), PR(−), Her2(0), Ki67(30%) | TN | II | Negative | TAC X6 | Negative | Non-pCR | BSG |
| 9 | IDC | cT2N1M0 | ER(−), PR(−), Her2(1+), Ki67(+50%) | TN | III | Negative | TAC X6 | Negative | Non-pCR | BSG |
| 10 | IDC | cT2N1M0 | ER(−), PR(−), Her2(3+), Ki67(30%) | HR-/HER2+ | III | Negative | TCbHP X6 | Negative | pCR | BSG |
| 11 | IDC | cT2N1M0 | ER(−), PR(−), Her2(3+), Ki67(30%) | HR-/HER2+ | II | Negative | TCbHP X6 | Negative | pCR | BSG |
| 12 | IDC | cT2N1M0 | ER(−), PR(−), Her2(3+), Ki67(30%+) | HR-/HER2+ | II | Negative | TCbHP X6 | Negative | pCR | BSG |
| 13 | IDC | cT2N3M0 | ER(−), PR(−), Her2(2+), Ki67(+ 10%) | TN | III | Positive | TAC X6 | Negative | pCR | Other |
| 14 | IDC | cT2N3M1 | ER(−), PR(−), Her2(3+), Ki67(10%) | HR-/HER2+ | II | Negative | TCbHP X6 | Negative | pCR | Other |
| 15 | IDC | cT2N1M0 | ER(2+ 90%), PR(2+), Her2(1+), Ki67(+ 10%) | Luminal B | II | Positive | TAC X6 | Negative | Non-pCR | Other |
| 16 | IDC | cT2N0M0 | ER(+5%), PR(+5%), Her2(3+), Ki67(30%) | HR+/HER2+ | III | Negative | TCbHP X6 | Negative | Non-pCR | Other |
| 17 | IDC | cT2N1M0 | ER(-), PR(−), Ki67(+50%), Her2(1+) | TN | II | Negative | AC-T X8 | Negative | Non-pCR | BSG |
| 18 | IDC | cT2N3M0 | ER (−), PR (−), Her2 (3+), Ki67(20%) | HR-/HER2+ | III | Positive | TCbHP X6 | Negative | Non-pCR | BSG |
| 19 | IDC | cT2N0M0 | ER(+), PR(−), Ki67(50%+), Her2(3+) | HR-/HER2+ | II | Negative | AC-TH X6 | Negative | Non-pCR | BSG |
| 20 | IDC | cT2N1M0 | ER(−), PR(−), HER-2(2+), Ki67(20%+) | TN | II | Positive | AC-T X8 | Positive | pCR | BSG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Chen, F.; Cao, H.; Zhu, W.; Deng, J.; Li, D.; Li, W.; Deng, J.; Zhong, Y.; Feng, H.; et al. Customised 3D-Printed Surgical Guide for Breast-Conserving Surgery after Neoadjuvant Chemotherapy and Its Clinical Application. Bioengineering 2023, 10, 1296. https://doi.org/10.3390/bioengineering10111296
Luo J, Chen F, Cao H, Zhu W, Deng J, Li D, Li W, Deng J, Zhong Y, Feng H, et al. Customised 3D-Printed Surgical Guide for Breast-Conserving Surgery after Neoadjuvant Chemotherapy and Its Clinical Application. Bioengineering. 2023; 10(11):1296. https://doi.org/10.3390/bioengineering10111296
Chicago/Turabian StyleLuo, Jie, Feng Chen, Hong Cao, Wei Zhu, Jian Deng, Dan Li, Wei Li, Junjie Deng, Yangyan Zhong, Haigang Feng, and et al. 2023. "Customised 3D-Printed Surgical Guide for Breast-Conserving Surgery after Neoadjuvant Chemotherapy and Its Clinical Application" Bioengineering 10, no. 11: 1296. https://doi.org/10.3390/bioengineering10111296