Immunotherapy in Acute Myeloid Leukemia: A Literature Review of Emerging Strategies
Abstract
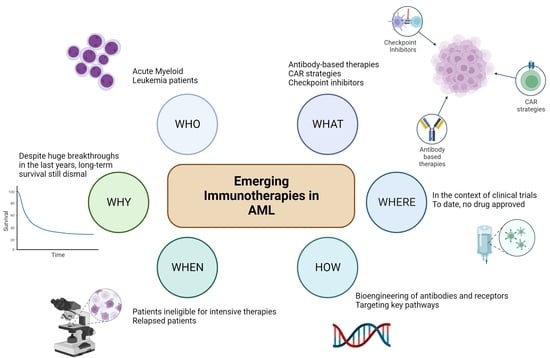
:1. Introduction
2. Antibody-Based Therapy
2.1. T-Cell-Binding Antibodies
2.2. NK-Cell-Binding Antibodies
| Drug (Phase of the Study) | Structure | Outcome | Year | Reference |
|---|---|---|---|---|
| Flotetuzumab (Phase I) | AntiCD3 × CD123 DART | ORR 22%, cCR 19% | 2018 | [47] |
| GTB-3550 (Phase I, dose escalation) | CD16/IL-15/CD33 TriKE | No Response | 2018 | [62] |
| AMG673 (Phase I, dose escalation) | AntiCD3 × CD33 BiTE | ORR 22.2%, cCR 3.7% | 2019 | [51] |
| AMG330 (Phase I, dose escalation) | AntiCD3 × CD33 BiTE | cCR 19% | 2020 | [50] |
| Vibecotamab (Phase I, dose escalation) | AntiCD3 × CD123 BiTE | cCR 13.7% | 2020 | [52] |
| Tepoditamab (Phase I, dose escalation) | AntiCD3 × CLEC12A BiTE | ORR 10.3% cCR 1.7% | 2020 | [57] |
| APVO436 (Phase I, dose escalation) | AntiCD3 × CD123 BiTE | CR 5.8% | 2021 | [54] |
| Flotetuzumab (Phase I/II) | AntiCD3 × CD123 DART | ORR 24%, cCR 20% | 2021 | [48] |
| GTB-3550 (Phase I) | CD16/IL-15/CD33 TriKE | ORR 25% | 2021 | [63] |
| APVO436 (Phase I) | AntiCD3 × CD123 BiTE | cCR 20% (monotherapy) cCR 33% (+MEC chemotherapy) cCR40% (+AZA/VEN) | 2022 | [55] |
3. Chimeric Antigen Receptor Strategies
3.1. CAR-T
3.2. CAR-NK
| Drug (Phase of the Study) | Structure | Outcome | Year | Reference |
|---|---|---|---|---|
| CD33-CAR-T (Phase I) | CAR-T × CD33 | No response * | 2015 | [67] |
| NKG2D-CAR-T (Phase I) | CAR-T × NKG2D | No response | 2019 | [69] |
| CD33-CAR-T (Phase I) | CAR-T × CD33 | No response | 2021 | [70] |
| CAR-T-38 (Phase I) | CAR-T × CD38 | ORR 100% * | 2021 | [71] |
| CAR-T-CLL1 (Phase I) | CAR-T × CLL1 | ORR 81.8% cCR 72.7% ** | 2021 | [78] |
| PRGN-3006 (Phase I, dose escalation) | CAR-T × CD33, Il-15 bound | ORR 0% in non-lymphodepleting cohort ORR 30% cCR 20% in lymphodepleting cohort | 2022 | [73] |
| CAR-T-123 (Phase I, dose escalation) | CAR-T × CD123 | ORR 16.7% CR 8.3% ** | 2022 | [75] |
| CAR-T-CLL1 (Phase I) | CAR-T × CLL1 | cCR 70% | 2022 | [77] |
| CAR-T-CLL1 (Phase I/II) | CAR-T × CLL1 | ORR 87.5% cCR 75% ** | 2022 | [79] |
| UCART123v1.2 + Alemtuzumab (Phase I, dose escalation) | CAR-T × CD123 | cCR 12.5% | 2022 | [74] |
| CD33-CAR-NK + Fludarabine/Cyclophosphamide (Phase I, dose escalation) | CAR-NK × CD33 | MRD-CR 60% | 2022 | [81] |
| CAR-T-CLL1 (Phase I) | 4-1-BB CAR-T × CLL1 CD28/CD27 CAR-T × CLL1 | CR 67% ** CR 75% ** | 2023 | [80] |
3.3. Future Directions
- CD7 (NCT04762485, NCT04033302), whose targeted CAR-T was able to efficiently kill CD7+ AML cells and CD7+ primary blasts of R/R-AML patients in vitro and significantly inhibit leukemia cell growth in a xenograft mouse model [82];
- CD19 (NCT04257175), occasionally expressed in AML blasts (especially in CBF-AML) [83];
- CD44v6 (NCT04097301), overexpressed in FLT3 and DNMT3A-mutated AML and already successfully targeted by CAR-T constructs in pre-clinical studies [84];
- CD70 (NCT04662294), expressed on most leukemic blasts but with little or no expression in normal bone marrow samples, is already targeted by the human monoclonal antibody Casatuzumab [85];
- FLT3 (NCT05023707, NCT03904069, NCT05017883), whose targeted CAR-T showed potent inhibition of leukemia proliferation in xenograft models [86];
- LILRB4 (alias ILT3) (NCT04803929), highly expressed in AML with monocytic differentiation and suspected to be involved in tumor escape, maintains an immunosuppressive milieu for tumor cells [87];
- CD276 (NCT04692948), expressed in AML with monocytic differentiation as well, whose targeted CAR-T exhibited efficient antigen-dependent cytotoxicity in vitro and in xenograft models of AML [88].
4. Checkpoint Inhibitors
4.1. SIRP/CD47 Pathway
4.2. PD1/PDL1 Pathway
4.3. TIM3/GAL-9 Pathway
4.4. CD27/CD70 Pathway
4.5. CTLA4/CD80-86 Pathway
| Drug (Phase of the Study) | Target | Population | Outcome | Year | Reference |
|---|---|---|---|---|---|
| Nivolumab + AZA (Phase Ib/II) | Anti-PD1 | 53 R/R AML | ORR 35% cCR 21% | 2017 | [112] |
| CC-90002 (Phase I) | Anti-CD47 | 24 R/R AML 4 R/R HR MDS | No Response | 2019 | [97] |
| Nivolumab + Cytarabine + Idarubicine (Phase II) | Anti-PD1 | 42 ND AML 2 HR MDS | ORR 77.2% | 2019 | [114] |
| Pembrolizumab + AZA (Phase II) | Anti-PD1 | 37 R/R AML 22 ND AML | ORR 17.2% cCR 13.8% ORR 58.8% cCR 47% | 2019 | [118] |
| Magrolimab + AZA (Phase I) | Anti-CD47 | 25 ND AML | ORR 69% cCR 56% | 2019 | [98] |
| Lemzoparlimab (Phase I) | Anti-CD47 | 5 R/R AML | MLFS 20% | 2020 | [101] |
| Sabatolimab + HMA (Phase I) | Anti-TIM3 | 50 ND or R/R AML | ORR 41% (DEC) ORR 27% (AZA) ORR 25% (DEC) | 2020 | [135] |
| Pembrolizumab + Cytarabine (Phase II) | Anti-PD1 | 37 R/R | ORR 46% cCR 38% | 2021 | [120] |
| Sabatolimab + HMA (Phase I/Ib) | Anti-TIM3 | 48 ND AML | ORR 40% | 2021 | [136] |
| Magrolimab + AZA (Phase I) | Anti-CD47 | 52 ND AML | ORR 65% cCR 62% | 2021 | [99] |
| Cusatuzumab + AZA/VEN (Phase I) | Anti-CD70 | 44 ND AML | cCR 92.9% | 2021 | [140] |
| Evorpacept + AZA/VEN (Phase I) | Anti-CD47 | 11 R/R AML 3 ND HR AML | ORR 44.4% ORR 100% | 2022 | [102] |
| Pembrolizumab + DEC (Phase II) | Anti-PD1 | 10 R/R AML | cCR 30% | 2022 | [119] |
| AZA ± Darvalumab (Phase II) | Anti-PD1 | 129 ND AML randomized to receive AZA with or without Darvalumab | ORR 31.3% vs. 35.4% | 2022 | [123] |
| Cusatuzumab + AZA (Phase I/II) | Anti-CD70 | 38 ND AML | cCR 37.9% | 2023 | [139] |
| Ipilimumab + DEC (Phase I) | Anti-CTLA4 | 25 postHSCT (23 R/R AML, 2 R/R MDS) 23 HSCT naive (15 AML, 8 MDS; 20 R/R 3 ND) | cCR 20% cCR 56.5% | 2023 | [145] |
4.6. Other Immune Checkpoint Targets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukemia |
| APL | acute promyelocytic leukemia |
| HMA | hypomethylating agents |
| VEN | Venetoclax |
| MRD | measurable residual disease |
| HSCT | hematopoietic stem cell transplantation |
| CBF-AML | core-Binding factor AML |
| CAR | chimeric antigen receptor |
| AZA | azacytidine |
| HDACIs | histone deacetylase inhibitors |
| BiTE | bispecific T-cell engager |
| ALL | acute lymphoblastic leukemia |
| scFv | single-chain variable fragment |
| DARTs | dual affinity retargeting antibodies |
| BiKE | bispecific killer engager |
| TriKE | trispecific killer engager |
| HSC | hematopoietic stem cells |
| CSR | cytokine release syndrome |
| BPDCN | blastic plasmacytoid dendritic cell neoplasms |
| ASH | American Society of Hematology |
| ORR | overall response rate |
| CR | complete response |
| CRi | complete response with incomplete count |
| MLFS | morphologic leukemia-free state |
| PR | partial response |
| cCR | composite complete response |
| IRR | infusion-related reactions |
| CML | chronic myeloid leukemia |
| R/R | relapsed/refractory |
| MEC | Mitoxantrone, Etoposide, and Cytarabine |
| NK | natural killer |
| NKG2D | natural killer group 2, member D |
| CMML | chronic myelomonocytic leukemia |
| TCR | T-cell receptor |
| GvHD | graft-vs-host disease |
| GvL | graft-vs-Leukemia |
| FLT3 | FMS-like receptor tyrosine kinase-3 |
| DNMT3A | DNA (cytosine-5-)-methyltransferase 3 alpha |
| LILRB4 | leukocyte immunoglobulin like receptor B4 |
| SIRPα | signal regulatory protein alpha |
| ND | newly diagnosed |
| TP53 | tumor protein P53 |
| PDL1 | programmed cell death 1 ligand 1 |
| HR | high risk |
| PD1 | programmed cell death protein 1 |
| IrAE | immune-related adverse events |
| TIM3 | T cell immunoglobulin and mucin domain 3 |
| Galectin-9 | (β-Galactoside-binding lectin 9) |
| DEC | Decitabine |
| CTLA4 | cytotoxic T-lymphocyte associated protein 4 |
| DLI | donor lymphocyte infusion |
| LAG-3 | lymphocyte activation gene-3 |
References
- Vardiman, J.W.; Harris, N.L.; Brunning, R.D. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 2002, 100, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E.; et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 2013, 369, 111–121. [Google Scholar] [CrossRef]
- Guarnera, L.; Ottone, T.; Fabiani, E.; Divona, M.; Savi, A.; Travaglini, S.; Falconi, G.; Panetta, P.; Rapanotti, M.C.; Voso, M.T. Atypical Rearrangements in APL-Like Acute Myeloid Leukemias: Molecular Characterization and Prognosis. Front. Oncol. 2022, 12, 871590. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.-N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef]
- Voso, M.T.; Ottone, T.; Lavorgna, S.; Venditti, A.; Maurillo, L.; Lo-Coco, F.; Buccisano, F. MRD in AML: The Role of New Techniques. Front. Oncol. 2019, 9, 655. [Google Scholar] [CrossRef]
- Tokaz, M.C.; Baldomero, H.; Cowan, A.J.; Saber, W.; Greinix, H.; Koh, M.B.C.; Kröger, N.; Mohty, M.; Galeano, S.; Okamoto, S.; et al. An Analysis of the Worldwide Utilization of Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia. Transplant. Cell Ther. 2023, 29, 279.e1–279.e10. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Short, N.J.; Fathi, A.T.; Marcucci, G.; Ravandi, F.; Tallman, M.; Wang, E.S.; Wei, A.H. Acute Myeloid Leukemia: Historical Perspective and Progress in Research and Therapy over 5 Decades. Clin. Lymphoma Myeloma Leuk. 2021, 21, 580–597. [Google Scholar] [CrossRef]
- Butturini, A.; Bortin, M.M.; Gale, R.P. Graft-versus-leukemia following bone marrow transplantation. Bone Marrow Transplant. 1987, 2, 233–242. [Google Scholar]
- Guarnera, L.; Santinelli, E.; Galossi, E.; Cristiano, A.; Fabiani, E.; Falconi, G.; Voso, M.T. Microenvironment in Acute Myeloid Leukemia: Focus on senescence mechanisms, therapeutic interactions and future directions. Exp. Hematol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, J.; Wu, J.; Carlson, A.L.; Silberstein, L.; Putheti, P.; Larocca, R.; Gao, W.; Saito, T.I.; Lo Celso, C.; Tsuyuzaki, H.; et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 2011, 474, 216–219. [Google Scholar] [CrossRef]
- Ros-Soto, J.; Snowden, J.A.; Szydlo, R.; Nicholson, E.; Madrigal, A.; Easdale, S.; Potter, M.; Ethell, M.; Anthias, C. Outcomes After Donor Lymphocyte Infusion in Patients with Hematological Malignancies: Donor Characteristics Matter. Transplant. Cell. Ther. 2022, 28, 183.e1–183.e8. [Google Scholar] [CrossRef]
- Sánchez-Abarca, L.I.; Gutierrez-Cosio, S.; Santamaría, C.; Caballero-Velazquez, T.; Blanco, B.; Herrero-Sánchez, C.; García, J.L.; Carrancio, S.; Hernández-Campo, P.; González, F.J.; et al. Immunomodulatory effect of 5-azacytidine (5-azaC): Potential role in the transplantation setting. Blood 2010, 115, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.L.; Choi, J.; Karpova, D.; Vij, K.; Ritchey, J.; Schroeder, M.A.; DiPersio, J.F. Azacitidine Mitigates Graft-versus-Host Disease via Differential Effects on the Proliferation of T Effectors and Natural Regulatory T Cells In Vivo. J. Immunol. 2017, 198, 3746–3754. [Google Scholar] [CrossRef]
- Grimm, J.; Simnica, D.; Jäkel, N.; Paschold, L.; Willscher, E.; Schulze, S.; Dierks, C.; Al-Ali, H.K.; Binder, M. Azacitidine-induced reconstitution of the bone marrow T cell repertoire is associated with superior survival in AML patients. Blood Cancer J. 2022, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; de Zoeten, E.F.; Greene, M.I.; Hancock, W.W. Immunomodulatory effects of deacetylase inhibitors: Therapeutic targeting of FOXP3+ regulatory T cells. Nat. Rev. Drug Discov. 2009, 8, 969–981. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Karagiannis, T.C. Regulation of immune responses by histone deacetylase inhibitors. ISRN Hematol. 2012, 2012, 690901. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Zhao, Y.; Huang, H. Immunomodulatory Effects of Histone Deacetylation Inhibitors in Graft-vs.-Host Disease After Allogeneic Stem Cell Transplantation. Front. Immunol. 2021, 12, 641910. [Google Scholar] [CrossRef]
- Mathew, N.R.; Baumgartner, F.; Braun, L.; O’Sullivan, D.; Thomas, S.; Waterhouse, M.; Müller, T.A.; Hanke, K.; Taromi, S.; Apostolova, P.; et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat. Med. 2018, 24, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Massumoto, C.; Sousa-Canavez, J.M.; Leite, K.R.M.; Camara-Lopes, L.H. Stabilization of acute myeloid leukemia with a dendritic cell vaccine. Hematol. Oncol. Stem Cell Ther. 2008, 1, 239–240. [Google Scholar] [CrossRef]
- Li, L.; Giannopoulos, K.; Reinhardt, P.; Tabarkiewicz, J.; Schmitt, A.; Greiner, J.; Rolinski, J.; Hus, I.; Dmoszynska, A.; Wiesneth, M.; et al. Immunotherapy for patients with acute myeloid leukemia using autologous dendritic cells generated from leukemic blasts. Int. J. Oncol. 2006, 28, 855–861. [Google Scholar] [CrossRef]
- Kitawaki, T.; Kadowaki, N.; Fukunaga, K.; Kasai, Y.; Maekawa, T.; Ohmori, K.; Itoh, T.; Shimizu, A.; Kuzushima, K.; Kondo, T.; et al. Cross-priming of CD8(+) T cells in vivo by dendritic cells pulsed with autologous apoptotic leukemic cells in immunotherapy for elderly patients with acute myeloid leukemia. Exp. Hematol. 2011, 39, 424–433.e2. [Google Scholar] [CrossRef]
- Dong, M.; Liang, D.; Li, Y.; Kong, D.; Kang, P.; Li, K.; Ping, C.; Zhang, Y.; Zhou, X.; Zhang, Y.; et al. Autologous dendritic cells combined with cytokine-induced killer cells synergize low-dose chemotherapy in elderly patients with acute myeloid leukaemia. J. Int. Med. Res. 2012, 40, 1265–1274. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Stone, R.M.; Uhl, L.; Neuberg, D.; Joyce, R.; Levine, J.D.; Arnason, J.; McMasters, M.; Luptakova, K.; Jain, S.; et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci. Transl. Med. 2016, 8, 368ra171. [Google Scholar] [CrossRef]
- Van de Loosdrecht, A.A.; Cloos, J.; Wagner, E.M.; Platzbecker, U.; Holderried, T.A.W.; van Elssen, J.; Giagounidis, A.; Lehmann, S.; van Zeeburg, H.; Rovers, J.; et al. Treatment with an Allogeneic Leukemia-Derived Dendritic Cell Vaccine in AML Patients Shows MRD Conversion and Improved Survival. Blood 2021, 138, 1274. [Google Scholar] [CrossRef]
- Kitawaki, T.; Kadowaki, N.; Fukunaga, K.; Kasai, Y.; Maekawa, T.; Ohmori, K.; Kondo, T.; Maekawa, R.; Takahara, M.; Nieda, M.; et al. A phase I/IIa clinical trial of immunotherapy for elderly patients with acute myeloid leukaemia using dendritic cells co-pulsed with WT1 peptide and zoledronate. Br. J. Haematol. 2011, 153, 796–799. [Google Scholar] [CrossRef]
- Shah, N.N.; Loeb, D.M.; Khuu, H.; Stroncek, D.; Ariyo, T.; Raffeld, M.; Delbrook, C.; Mackall, C.L.; Wayne, A.S.; Fry, T.J. Induction of Immune Response after Allogeneic Wilms’ Tumor 1 Dendritic Cell Vaccination and Donor Lymphocyte Infusion in Patients with Hematologic Malignancies and Post-Transplantation Relapse. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Barbullushi, K.; Rampi, N.; Serpenti, F.; Sciumè, M.; Fabris, S.; De Roberto, P.; Fracchiolla, N.S. Vaccination Therapy for Acute Myeloid Leukemia: Where Do We Stand? Cancers 2022, 14, 2994. [Google Scholar] [CrossRef] [PubMed]
- Rautenberg, C.; Germing, U.; Haas, R.; Kobbe, G.; Schroeder, T. Relapse of Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: Prevention, Detection, and Treatment. Int. J. Mol. Sci. 2019, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Huehls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 2015, 93, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Mocquot, P.; Mossazadeh, Y.; Lapierre, L.; Pineau, F.; Despas, F. The pharmacology of blinatumomab: State of the art on pharmacodynamics, pharmacokinetics, adverse drug reactions and evaluation in clinical trials. J. Clin. Pharm. Ther. 2022, 47, 1337–1351. [Google Scholar] [CrossRef]
- Einsele, H.; Borghaei, H.; Orlowski, R.Z.; Subklewe, M.; Roboz, G.J.; Zugmaier, G.; Kufer, P.; Iskander, K.; Kantarjian, H.M. The BiTE (bispecific T-cell engager) platform: Development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer 2020, 126, 3192–3201. [Google Scholar] [CrossRef]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK cells and ILCs in tumor immunotherapy. Mol. Asp. Med. 2021, 80, 100870. [Google Scholar] [CrossRef]
- Ehninger, A.; Kramer, M.; Röllig, C.; Thiede, C.; Bornhäuser, M.; von Bonin, M.; Wermke, M.; Feldmann, A.; Bachmann, M.; Ehninger, G.; et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014, 4, e218. [Google Scholar] [CrossRef] [PubMed]
- Paterno, G.; Guarnera, L.; Palmieri, R.; Del Prete, V.; Bonanni, F.; Buzzatti, E.; Moretti, F.; Casciani, P.; Savi, A.; Di Cave, D.; et al. Pneumocystis jirovecii pneumonia in patients with previously untreated acute myeloid leukaemia. Mycoses 2022, 65, 233–238. [Google Scholar] [CrossRef]
- Guarnera, L.; Trotta, G.E.; Boldrini, V.; Cardillo, L.; Cerroni, I.; Mezzanotte, V.; Pasqualone, G.; Savi, A.; Borsellino, B.; Buzzatti, E.; et al. Fever of Unknown Origin and Multidrug Resistant Organism Colonization in AML Patients. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023013. [Google Scholar] [CrossRef]
- Cattaneo, C.; Di Blasi, R.; Skert, C.; Candoni, A.; Martino, B.; Di Renzo, N.; Delia, M.; Ballanti, S.; Marchesi, F.; Mancini, V.; et al. Bloodstream infections in haematological cancer patients colonized by multidrug-resistant bacteria. Ann. Hematol. 2018, 97, 1717–1726. [Google Scholar] [CrossRef]
- McKoy, J.M.; Angelotta, C.; Bennett, C.L.; Tallman, M.S.; Wadleigh, M.; Evens, A.M.; Kuzel, T.M.; Trifilio, S.M.; Raisch, D.W.; Kell, J.; et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): An overview from the research on adverse drug events and reports (RADAR) project. Leuk. Res. 2007, 31, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Lane, A.A.; Sweet, K.L.; Stein, A.S.; Vasu, S.; Blum, W.; Rizzieri, D.A.; Wang, E.S.; Duvic, M.; Sloan, J.M.; et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N. Engl. J. Med. 2019, 380, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.A.; Stein, A.S.; Garcia, J.S.; Garzon, J.L.; Galinsky, I.; Luskin, M.R.; Stone, R.M.; Winer, E.S.; Leonard, R.; Mughal, T.I.; et al. Safety and Efficacy of Combining Tagraxofusp (SL-401) with Azacitidine or Azacitidine and Venetoclax in a Phase 1b Study for CD123 Positive AML, MDS, or BPDCN. Blood 2021, 138, 2346. [Google Scholar] [CrossRef]
- Green, S.D.; Parkin, B.; Lai, C.; Gojo, I.; Foster, M.C.; Zeidner, J.F. Phase II Study of Tagraxofusp in Newly Diagnosed Secondary Acute Myeloid Leukemia (S-AML) after Previous Exposure to Hypomethylating Agents (TAGALONG Study). Blood 2022, 140, 11749–11750. [Google Scholar] [CrossRef]
- Uy, G.L.; Rettig, M.P.; Vey, N.; Godwin, J.; Foster, M.C.; Rizzieri, D.A.; Arellano, M.L.; Topp, M.S.; Huls, G.; Jongen-Lavrencic, M.; et al. Phase 1 Cohort Expansion of Flotetuzumab, a CD123×CD3 Bispecific Dart® Protein in Patients with Relapsed/Refractory Acute Myeloid Leukemia (AML). Blood 2018, 132, 764. [Google Scholar] [CrossRef]
- Uy, G.L.; Aldoss, I.; Foster, M.C.; Sayre, P.H.; Wieduwilt, M.J.; Advani, A.S.; Godwin, J.E.; Arellano, M.L.; Sweet, K.L.; Emadi, A.; et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 2021, 137, 751–762. [Google Scholar] [CrossRef]
- Ravandi, F.; Stein, A.S.; Kantarjian, H.M.; Walter, R.B.; Paschka, P.; Jongen-Lavrencic, M.; Ossenkoppele, G.J.; Yang, Z.; Mehta, B.; Subklewe, M. A Phase 1 First-in-Human Study of AMG 330, an Anti-CD33 Bispecific T-Cell Engager (BiTE®) Antibody Construct, in Relapsed/Refractory Acute Myeloid Leukemia (R/R AML). Blood 2018, 132, 25. [Google Scholar] [CrossRef]
- Ravandi, F.; Walter, R.B.; Subklewe, M.; Buecklein, V.; Jongen-Lavrencic, M.; Paschka, P.; Ossenkoppele, G.J.; Kantarjian, H.M.; Hindoyan, A.; Agarwal, S.K.; et al. Updated results from phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule, in patients with relapsed/refractory acute myeloid leukemia (R/R AML). J. Clin. Oncol. 2020, 38, 7508. [Google Scholar] [CrossRef]
- Subklewe, M.; Stein, A.; Walter, R.B.; Bhatia, R.; Wei, A.H.; Ritchie, D.; Bücklein, V.; Vachhani, P.; Dai, T.; Hindoyan, A.; et al. Preliminary Results from a Phase 1 First-in-Human Study of AMG 673, a Novel Half-Life Extended (HLE) Anti-CD33/CD3 BiTE® (Bispecific T-Cell Engager) in Patients with Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML). Blood 2019, 134, 833. [Google Scholar] [CrossRef]
- Ravandi, F.; Bashey, A.; Stock, W.; Foran, J.M.; Mawad, R.; Egan, D.; Blum, W.; Yang, A.; Pastore, A.; Johnson, C.; et al. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of Vibecotamab (XmAb14045), a CD123 x CD3 T Cell-Engaging Bispecific Antibody; Initial Results of a Phase 1 Study. Blood 2020, 136, 4–5. [Google Scholar] [CrossRef]
- Short, N.J.; Bachireddy, P.; Huang, X.; Hwang, H.; Leng, X.; Lee, J.; Nguyen, D.; Garcia-Manero, G.; Dinardo, C.D.; Borthakur, G.; et al. A phase II study of vibecotamab, a CD3-CD123 bispecific T-cell engaging antibody, for MRD-positive AML and MDS after hypomethylating agent failure. J. Clin. Oncol. 2023, 41, TPS7076. [Google Scholar] [CrossRef]
- Uckun, F.M.; Lin, T.L.; Mims, A.S.; Patel, P.; Lee, C.; Shahidzadeh, A.; Shami, P.J.; Cull, E.; Cogle, C.R.; Watts, J. A Clinical Phase 1B Study of the CD3xCD123 Bispecific Antibody APVO436 in Patients with Relapsed/Refractory Acute Myeloid Leukemia or Myelodysplastic Syndrome. Cancers 2021, 13, 4113. [Google Scholar] [CrossRef]
- Watts, J.; Maris, M.; Lin, T.L.; Patel, P.; Madanat, Y.F.; Cogle, C.R.; Borthakur, G.; Huebner, D.; Khaskhely, N.; Bonham, L.; et al. Updated Results from a Phase 1 Study of APVO436, a Novel Bispecific Anti-CD123 x Anti-CD3 AdaptirTM Molecule, in Relapsed/Refractory Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood 2022, 140, 6204–6205. [Google Scholar] [CrossRef]
- Morsink, L.M.; Walter, R.B.; Ossenkoppele, G.J. Prognostic and therapeutic role of CLEC12A in acute myeloid leukemia. Blood Rev. 2019, 34, 26–33. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Cortes, J.; Huls, G.; Venditti, A.; Breems, D.; De Botton, S.; Deangelo, D.; van de Loosdrecht, A.; Jongen-Lavrencic, M.; Borthakur, G.; et al. Update from the Ongoing Phase I Multinational Study of MCLA-117, a Bispecific CLEC12A X CD3 T-Cell Engager, in Patients (Pts) with Acute Myelogenous Leukemia (AML); European Hematology Association: Hague, The Netherlands, 2020. [Google Scholar]
- Mehta, N.K.; Pfluegler, M.; Meetze, K.; Li, B.; Sindel, I.; Vogt, F.; Marklin, M.; Heitmann, J.S.; Kauer, J.; Osburg, L.; et al. A novel IgG-based FLT3xCD3 bispecific antibody for the treatment of AML and B-ALL. J. Immunother. Cancer 2022, 10, e003882. [Google Scholar] [CrossRef]
- Wiernik, A.; Foley, B.; Zhang, B.; Verneris, M.R.; Warlick, E.; Gleason, M.K.; Ross, J.A.; Luo, X.; Weisdorf, D.J.; Walcheck, B.; et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin. Cancer Res. 2013, 19, 3844–3855. [Google Scholar] [CrossRef]
- Gleason, M.K.; Ross, J.A.; Warlick, E.D.; Lund, T.C.; Verneris, M.R.; Wiernik, A.; Spellman, S.; Haagenson, M.D.; Lenvik, A.J.; Litzow, M.R.; et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 2014, 123, 3016–3026. [Google Scholar] [CrossRef]
- Reusing, S.B.; Vallera, D.A.; Manser, A.R.; Vatrin, T.; Bhatia, S.; Felices, M.; Miller, J.S.; Uhrberg, M.; Babor, F. CD16xCD33 Bispecific Killer Cell Engager (BiKE) as potential immunotherapeutic in pediatric patients with AML and biphenotypic ALL. Cancer Immunol. Immunother. 2021, 70, 3701–3708. [Google Scholar] [CrossRef]
- Erica, D.; Warlick, M.; Daniel, J.; Weisdorf, M.; Daniel, A.; Vallera, P.; Wangen, R.; Dixie Lewis, R.N.; Knox, J.; Schroeder, M.; et al. GTB-3550 TriKETM for the Treatment of High-Risk Myelodysplastic Syndromes (MDS) and Refractory/Relapsed Acute Myeloid Leukemia (AML) Safely Drives Natural Killer (NK) Cell Proliferation at Initial dose Cohorts. Blood 2020, 136, 7–8. [Google Scholar]
- Felices, M.; Warlick, E.; Juckett, M.; Weisdorf, D.; Vallera, D.; Miller, S.; Wangen, R.; Lewis, D.; Knox, J.; Schroeder, M.; et al. GTB-3550 tri-specific killer engager TriKETM drives NK cells expansion and cytotoxicity in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) patients. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- Hughes-Parry, H.E.; Cross, R.S.; Jenkins, M.R. The Evolving Protein Engineering in the Design of Chimeric Antigen Receptor T Cells. Int. J. Mol. Sci. 2019, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, U.; Khan, Z.; Ualiyeva, D.; Amissah, O.B.; Noor, Z.; Khan, A.; Zaman, N.; Khan, M.; Khan, A.; Ali, B. Chimeric antigen receptor T cell structure, its manufacturing, and related toxicities Chimeric antigen receptor T cell structure, its manufacturing, and related toxicities; A comprehensive review. Adv. Cancer Biol.-Metastasis 2022, 4, 100035. [Google Scholar] [CrossRef]
- Tang, H.K.C.; Wang, B.; Tan, H.X.; Sarwar, M.A.; Baraka, B.; Shafiq, T.; Rao, A.R. CAR T-Cell Therapy for Cancer: Latest Updates and Challenges, with a Focus on B-Lymphoid Malignancies and Selected Solid Tumours. Cells 2023, 12, 1586. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Lv, H.; Han, Q.; Fan, H.; Guo, B.; Wang, L.; Han, W. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol. Ther. 2015, 23, 184–191. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Wu, M.; Peng, G.; He, Y.; Wan, N.; Zeng, Y. Targeting the NKG2D/NKG2D-L axis in acute myeloid leukemia. Biomed. Pharmacother. 2021, 137, 111299. [Google Scholar] [CrossRef]
- Baumeister, S.H.; Murad, J.; Werner, L.; Daley, H.; Trebeden-Negre, H.; Gicobi, J.K.; Schmucker, A.; Reder, J.; Sentman, C.L.; Gilham, D.E.; et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol. Res. 2019, 7, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Tambaro, F.P.; Singh, H.; Jones, E.; Rytting, M.; Mahadeo, K.M.; Thompson, P.; Daver, N.; DiNardo, C.; Kadia, T.; Garcia-Manero, G.; et al. Autologous CD33-CAR-T cells for treatment of relapsed/refractory acute myelogenous leukemia. Leukemia 2021, 35, 3282–3286. [Google Scholar] [CrossRef]
- Hu, N.; Gao, C.; Zhang, Y.; Teng, G.; Wang, Y.; Du, C.; Bai, J. Targeted Therapy in CD38 + Acute Myeloid Leukemia. Blood 2021, 138, 4425. [Google Scholar] [CrossRef]
- Glisovic-Aplenc, T.; Diorio, C.; Chukinas, J.A.; Veliz, K.; Shestova, O.; Shen, F.; Nunez-Cruz, S.; Vincent, T.L.; Miao, F.; Milone, M.C.; et al. CD38 as a pan-hematologic target for chimeric antigen receptor T cells. Blood Adv. 2023, 7, 4418–4430. [Google Scholar] [CrossRef]
- Sallman, D.A.; Elmariah, H.; Sweet, K.; Mishra, A.; Cox, C.A.; Chakaith, M.; Semnani, R.; Shehzad, S.; Anderson, A.; Sabzevari, H.; et al. Phase 1/1b Safety Study of Prgn-3006 Ultracar-T in Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia and Higher Risk Myelodysplastic Syndromes. Blood 2022, 140, 10313–10315. [Google Scholar] [CrossRef]
- Sallman, D.A.; DeAngelo, D.J.; Pemmaraju, N.; Dinner, S.; Gill, S.; Olin, R.L.; Wang, E.S.; Konopleva, M.; Stark, E.; Korngold, A.; et al. Ameli-01: A Phase I Trial of UCART123v1.2, an Anti-CD123 Allogeneic CAR-T Cell Product, in Adult Patients with Relapsed or Refractory (R/R) CD123+ Acute Myeloid Leukemia (AML). Blood 2022, 140, 2371–2373. [Google Scholar] [CrossRef]
- Naik, S.; Madden, R.M.; Lipsitt, A.; Lockey, T.; Bran, J.; Rubnitz, J.E.; Klco, J.; Shulkin, B.; Patil, S.L.; Schell, S.; et al. Safety and Anti-Leukemic Activity of CD123-CAR T Cells in Pediatric Patients with AML: Preliminary Results from a Phase 1 Trial. Blood 2022, 140, 4584–4585. [Google Scholar] [CrossRef]
- Zhang, H.; Gan, W.-T.; Hao, W.-G.; Wang, P.-F.; Li, Z.-Y.; Chang, L.-J. Successful Anti-CLL1 CAR T-Cell Therapy in Secondary Acute Myeloid Leukemia. Front. Oncol. 2020, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, M.; Sun, R.; Lyu, H.; Xiao, X.; Zhang, X.; Li, F.; Xie, D.; Xiong, X.; Wang, J.; et al. First-in-human phase I study of CLL-1 CAR-T cells in adults with relapsed/refractory acute myeloid leukemia. J. Hematol. Oncol. 2022, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bu, C.; Peng, Z.; Luo, M.; Li, C. The efficacy and safety of anti-CLL1 based CAR-T cells in children with relapsed or refractory acute myeloid leukemia: A multicenter interim analysis. J. Clin. Oncol. 2021, 39, 10000. [Google Scholar] [CrossRef]
- Zhang, H.; Bu, C.; Peng, Z.; Li, G.; Zhou, Z.; Ding, W.; Zheng, Y.; He, Y.; Hu, Z.; Pei, K.; et al. Characteristics of anti-CLL1 based CAR-T therapy for children with relapsed or refractory acute myeloid leukemia: The multi-center efficacy and safety interim analysis. Leukemia 2022, 36, 2596–2604. [Google Scholar] [CrossRef]
- Pei, K.; Xu, H.; Wang, P.; Gan, W.; Hu, Z.; Su, X.; Zhang, H.; He, Y. Anti-CLL1-based CAR T-cells with 4-1-BB or CD28/CD27 stimulatory domains in treating childhood refractory/relapsed acute myeloid leukemia. Cancer Med. 2023, 12, 9655–9661. [Google Scholar] [CrossRef]
- Huang, R.; Wen, Q.; Wang, X.; Yan, H.; Ma, Y.; Mai-Hong, W.; Han, X.; Gao, L.; Gao, L.; Zhang, C.; et al. Off-the-Shelf CD33 CAR-NK Cell Therapy for Relapse/Refractory AML: First-in-Human, Phase I Trial. Blood 2022, 140, 7450–7451. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Wen, S.; Kuang, N.; Zhang, X.; Li, J.; Wang, F. Naturally selected CD7 CAR-T therapy without genetic editing demonstrates significant antitumour efficacy against relapsed and refractory acute myeloid leukaemia (R/R-AML). J. Transl. Med. 2022, 20, 600. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.; Dharmadhikari, A.V.; Sait, S.N.J.; Deeb, G.; Wallace, P.K.; Thompson, J.E.; Wang, E.S.; Wetzler, M. CD19 expression in acute leukemia is not restricted to the cytogenetically aberrant populations. Leuk. Lymphoma 2013, 54, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Huang, H.; Tang, Y.; Li, Q.; Wang, J.; Li, D.; Zhong, Z.; Zou, P.; You, Y.; Cao, Y.; et al. CD44v6 chimeric antigen receptor T cell specificity towards AML with FLT3 or DNMT3A mutations. Clin. Transl. Med. 2022, 12, e1043. [Google Scholar] [CrossRef] [PubMed]
- Riether, C.; Pabst, T.; Höpner, S.; Bacher, U.; Hinterbrandner, M.; Banz, Y.; Müller, R.; Manz, M.G.; Gharib, W.H.; Francisco, D.; et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat. Med. 2020, 26, 1459–1467. [Google Scholar] [CrossRef]
- Niswander, L.M.; Graff, Z.T.; Chien, C.D.; Chukinas, J.A.; Meadows, C.A.; Leach, L.C.; Loftus, J.P.; Kohler, M.E.; Tasian, S.K.; Fry, T.J. Potent preclinical activity of FLT3-directed chimeric antigen receptor T-cell immunotherapy against FLT3- mutant acute myeloid leukemia and KMT2A-rearranged acute lymphoblastic leukemia. Haematologica 2023, 108, 457–471. [Google Scholar] [CrossRef]
- Shao, R.; Li, Z.; Xin, H.; Jiang, S.; Zhu, Y.; Liu, J.; Huang, R.; Xu, K.; Shi, X. Biomarkers as targets for CAR-T/NK cell therapy in AML. Biomark. Res. 2023, 11, 65. [Google Scholar] [CrossRef]
- Lichtman, E.I.; Du, H.; Shou, P.; Song, F.; Suzuki, K.; Ahn, S.; Li, G.; Ferrone, S.; Su, L.; Savoldo, B.; et al. Preclinical Evaluation of B7-H3-specific Chimeric Antigen Receptor T Cells for the Treatment of Acute Myeloid Leukemia. Clin. Cancer Res. 2021, 27, 3141–3153. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Kuzume, A.; Chi, S.; Yamauchi, N.; Minami, Y. Immune-Checkpoint Blockade Therapy in Lymphoma. Int. J. Mol. Sci. 2020, 21, 5456. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, H.; Yu, J.; Tian, W.; Song, Y. Targeting CD47 for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Komori, S.; Saito, Y.; Nishimura, T.; Respatika, D.; Endoh, H.; Yoshida, H.; Sugihara, R.; Iida-Norita, R.; Afroj, T.; Takai, T.; et al. CD47 promotes peripheral T cell survival by preventing dendritic cell-mediated T cell necroptosis. Proc. Natl. Acad. Sci. USA 2023, 120, e2304943120. [Google Scholar] [CrossRef]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.J.; van Rooijen, N.; Weissman, I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Lai, B.; Zhou, X.; Yang, S.; Ge, Q.; Zhou, M.; Shi, C.; Xu, Z.; Ouyang, G. The Differential Expression of CD47 may be Related to the Pathogenesis From Myelodysplastic Syndromes to Acute Myeloid Leukemia. Front. Oncol. 2022, 12, 872999. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Jamieson, C.H.M.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef]
- Tseng, D.; Volkmer, J.-P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108. [Google Scholar] [CrossRef]
- Zeidan, A.M.; DeAngelo, D.J.; Palmer, J.M.; Seet, C.S.; Tallman, M.S.; Wei, X.; Li, Y.F.; Hock, N.; Burgess, M.R.; Hege, K.; et al. A Phase I Study of CC-90002, a Monoclonal Antibody Targeting CD47, in Patients with Relapsed and/or Refractory (R/R) Acute Myeloid Leukemia (AML) and High-Risk Myelodysplastic Syndromes (MDS): Final Results. Blood 2019, 134, 1320. [Google Scholar] [CrossRef]
- Sallman, D.A.; Asch, A.S.; Al Malki, M.M.; Lee, D.J.; Donnellan, W.B.; Marcucci, G.; Kambhampati, S.; Daver, N.G.; Garcia-Manero, G.; Komrokji, R.S.; et al. The First-in-Class Anti-CD47 Antibody Magrolimab (5F9) in Combination with Azacitidine Is Effective in MDS and AML Patients: Ongoing Phase 1b Results. Blood 2019, 134, 569. [Google Scholar] [CrossRef]
- Sallman, D.; Asch, A.; Kambhampati, S.; Al Malki, M.; Zeidner, J.; Donnellan, W.; Lee, D.; Vyas, P.; Jeyakumar, D.; Mannis, G.; et al. AML-196: The First-in-Class Anti-CD47 Antibody Magrolimab in Combination with Azacitidine Is Well Tolerated and Effective in AML Patients: Phase 1b Results. Clin. Lymphoma Myeloma Leuk. 2021, 21, S290. [Google Scholar] [CrossRef]
- Gilead To Discontinue Phase 3 ENHANCE Study of Magrolimab Plus Azacitidine in Higher-Risk MDS; Gilead Sciences: Foster City, CA, USA, 2023.
- Qi, J.; Li, J.; Jiang, B.; Jiang, B.; Liu, H.; Cao, X.; Zhang, M.; Meng, Y.; Xiaoyu, M.A.; Jia, Y.; et al. A Phase I/IIa Study of Lemzoparlimab, a Monoclonal Antibody Targeting CD47, in Patients with Relapsed and/or Refractory Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS): Initial Phase I Results. Blood 2020, 136, 30–31. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Przespolewski, A.; Abaza, Y.; Byrne, M.; Fong, A.P.; Jin, F.; Forgie, A.J.; Tsiatis, A.C.; Guan, S.; Erba, H.P. Evorpacept (ALX148), a CD47-Blocking Myeloid Checkpoint Inhibitor, in Combination with Azacitidine and Venetoclax in Patients with Acute Myeloid Leukemia (ASPEN-05): Results from Phase 1a Dose Escalation Part. Blood 2022, 140, 9046–9047. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Riley, J.L. PD-1 signaling in primary T cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Brodská, B.; Otevřelová, P.; Šálek, C.; Fuchs, O.; Gašová, Z.; Kuželová, K. High PD-L1 Expression Predicts for Worse Outcome of Leukemia Patients with Concomitant NPM1 and FLT3 Mutations. Int. J. Mol. Sci. 2019, 20, 2823. [Google Scholar] [CrossRef]
- Yang, X.; Ma, L.; Zhang, X.; Huang, L.; Wei, J. Targeting PD-1/PD-L1 pathway in myelodysplastic syndromes and acute myeloid leukemia. Exp. Hematol. Oncol. 2022, 11, 11. [Google Scholar] [CrossRef]
- Williams, P.; Basu, S.; Garcia-Manero, G.; Hourigan, C.S.; Oetjen, K.A.; Cortes, J.E.; Ravandi, F.; Jabbour, E.J.; Al-Hamal, Z.; Konopleva, M.; et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer 2019, 125, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wu, J.; Li, C.-G.; Jiang, H.-W.; Xu, M.; Du, M.; Yin, Z.; Mei, H.; Hu, Y. Characterization of Immune Dysfunction and Identification of Prognostic Immune-Related Risk Factors in Acute Myeloid Leukemia. Clin. Cancer Res. 2020, 26, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Damiani, D.; Tiribelli, M. Checkpoint Inhibitors in Acute Myeloid Leukemia. Biomedicines 2023, 11, 1724. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.G.; Basu, S.; Garcia-Manero, G.; Cortes, J.E.; Ravandi, F.; Jabbour, E.; Hendrickson, S.; Brandt, M.; Pierce, S.; Gordon, T.; et al. Phase IB/II study of nivolumab with azacytidine (AZA) in patients (pts) with relapsed AML. J. Clin. Oncol. 2017, 35, 7026. [Google Scholar] [CrossRef]
- Ravandi, F.; Daver, N.; Garcia-Manero, G.; Benton, C.B.; Thompson, P.A.; Borthakur, G.; Kadia, T.; Boddu, P.C.; Alvarado, Y.; Jabbour, E.J.; et al. Phase 2 Study of Combination of Cytarabine, Idarubicin, and Nivolumab for Initial Therapy of Patients with Newly Diagnosed Acute Myeloid Leukemia. Blood 2017, 130, 815. [Google Scholar] [CrossRef]
- Ravandi, F.; Assi, R.; Daver, N.; Benton, C.B.; Kadia, T.; Thompson, P.A.; Borthakur, G.; Alvarado, Y.; Jabbour, E.J.; Konopleva, M.; et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A single-arm, phase 2 study. Lancet Haematol. 2019, 6, e480–e488. [Google Scholar] [CrossRef] [PubMed]
- Kadia, T.M.; Cortes, J.E.; Ghorab, A.; Ravandi, F.; Jabbour, E.; Daver, N.G.; Alvarado, Y.; Ohanian, M.; Konopleva, M.; Kantarjian, H.M. Nivolumab (Nivo) maintenance (maint) in high-risk (HR) acute myeloid leukemia (AML) patients. J. Clin. Oncol. 2018, 36, 7014. [Google Scholar] [CrossRef]
- Reville, P.K.; Kantarjian, H.M.; Ravandi, F.; Jabbour, E.; DiNardo, C.D.; Daver, N.; Pemmaraju, N.; Ohanian, M.; Alvarado, Y.; Xiao, L.; et al. Nivolumab maintenance in high-risk acute myeloid leukemia patients: A single-arm, open-label, phase II study. Blood Cancer J. 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sharon, E.; Karrison, T.G.; Zha, Y.; Fulton, N.; Streicher, H.; Sweet, K.; Yaghmour, G.; Liu, J.J.; Jonas, B.A.; et al. Randomized Phase II Study to Assess the Role of Nivolumab As Single Agent to Eliminate Minimal Residual Disease and Maintain Remission in Acute Myelogenous Leukemia (AML) Patients after Chemotherapy (NCI9706 protocol; REMAIN Trial). Blood 2022, 140, 1716–1719. [Google Scholar] [CrossRef]
- Gojo, I.; Stuart, R.K.; Webster, J.; Blackford, A.; Varela, J.C.; Morrow, J.; DeZern, A.E.; Foster, M.C.; Levis, M.J.; Coombs, C.C.; et al. Multi-Center Phase 2 Study of Pembroluzimab (Pembro) and Azacitidine (AZA) in Patients with Relapsed/Refractory Acute Myeloid Leukemia (AML) and in Newly Diagnosed (≥65 Years) AML Patients. Blood 2019, 134, 832. [Google Scholar] [CrossRef]
- Goswami, M.; Gui, G.; Dillon, L.W.; Lindblad, K.E.; Thompson, J.; Valdez, J.; Kim, D.-Y.; Ghannam, J.Y.; Oetjen, K.A.; Destefano, C.B.; et al. Pembrolizumab and decitabine for refractory or relapsed acute myeloid leukemia. J. Immunother. Cancer 2022, 10, e003392. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Vincent, B.G.; Ivanova, A.; Moore, D.; McKinnon, K.P.; Wilkinson, A.D.; Mukhopadhyay, R.; Mazziotta, F.; Knaus, H.A.; Foster, M.C.; et al. Phase II Trial of Pembrolizumab after High-Dose Cytarabine in Relapsed/Refractory Acute Myeloid Leukemia. Blood Cancer Discov. 2021, 2, 616–629. [Google Scholar] [CrossRef]
- Tschernia, N.P.; Kumar, V.; Moore, D.T.; Vincent, B.G.; Coombs, C.C.; Van Deventer, H.; Foster, M.C.; DeZern, A.E.; Luznik, L.; Riches, M.L.; et al. Safety and Efficacy of Pembrolizumab Prior to Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia. Transplant. Cell. Ther. 2021, 27, 1021.e1–1021.e5. [Google Scholar] [CrossRef]
- Solomon, S.R.; Solh, M.M.; Morris, L.E.; Holland, H.K.; Bachier-Rodriguez, L.; Zhang, X.; Guzowski, C.; Jackson, K.C.; Brown, S.; Bashey, A. Phase 2 Study of PD-1 blockade following autologous transplantation for patients with AML ineligible for allogeneic transplant. Blood Adv. 2023, 7, 5215–5224. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Boss, I.; Beach, C.L.; Copeland, W.B.; Thompson, E.; Fox, B.A.; Hasle, V.E.; Hellmann, A.; Taussig, D.C.; Tormo, M.; et al. A randomized phase 2 trial of azacitidine with or without durvalumab as first-line therapy for older patients with AML. Blood Adv. 2022, 6, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Ndhlovu, L.C.; Lopez-Vergès, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119, 3734–3743. [Google Scholar] [CrossRef] [PubMed]
- Sakuishi, K.; Ngiow, S.F.; Sullivan, J.M.; Teng, M.W.L.; Kuchroo, V.K.; Smyth, M.J.; Anderson, A.C. TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology 2013, 2, e23849. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Li, L.; Fu, R.; Wang, H.; Jiang, H.; Yue, L.; Zhang, W.; Liu, H.; Ruan, E.; Qu, W.; et al. Elevated TIM3+ hematopoietic stem cells in untreated myelodysplastic syndrome displayed aberrant differentiation, overproliferation and decreased apoptosis. Leuk. Res. 2014, 38, 714–721. [Google Scholar] [CrossRef]
- Mohamed, M.M.I.; Aref, S.; Agdar, M.A.; Mabed, M.; El-Sokkary, A.M.A. Leukemic Stem Cell (CD34(+)/CD38(-)/TIM3(+)) Frequency in Patients with Acute Myeloid Leukemia: Clinical Implications. Clin. Lymphoma. Myeloma Leuk. 2021, 21, 508–513. [Google Scholar] [CrossRef]
- Tan, J.; Yu, Z.; Huang, J.; Chen, Y.; Huang, S.; Yao, D.; Xu, L.; Lu, Y.; Chen, S.; Li, Y. Increased PD-1+Tim-3+ exhausted T cells in bone marrow may influence the clinical outcome of patients with AML. Biomark. Res. 2020, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, J.; Claxton, D.F.; Ehmann, W.C.; Rybka, W.B.; Zhu, L.; Zeng, H.; Schell, T.D.; Zheng, H. PD-1(hi)TIM-3(+) T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation. Blood Cancer J. 2015, 5, e330. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Yu, X.; Zhu, Y.; Ma, C.; Xia, R.; Ma, J.; Gu, C.; Ye, L.; Wu, D. Tim-3 is highly expressed in T cells in acute myeloid leukemia and associated with clinicopathological prognostic stratification. Int. J. Clin. Exp. Pathol. 2014, 7, 6880–6888. [Google Scholar]
- Zahran, A.M.; Mohammed Saleh, M.F.; Sayed, M.M.; Rayan, A.; Ali, A.M.; Hetta, H.F. Up-regulation of regulatory T cells, CD200 and TIM3 expression in cytogenetically normal acute myeloid leukemia. Cancer Biomark. 2018, 22, 587–595. [Google Scholar] [CrossRef]
- Darwish, N.H.E.; Sudha, T.; Godugu, K.; Elbaz, O.; Abdelghaffar, H.A.; Hassan, E.E.A.; Mousa, S.A. Acute myeloid leukemia stem cell markers in prognosis and targeted therapy: Potential impact of BMI-1, TIM-3 and CLL-1. Oncotarget 2016, 7, 57811–57820. [Google Scholar] [CrossRef]
- Kikushige, Y.; Shima, T.; Takayanagi, S.; Urata, S.; Miyamoto, T.; Iwasaki, H.; Takenaka, K.; Teshima, T.; Tanaka, T.; Inagaki, Y.; et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell 2010, 7, 708–717. [Google Scholar] [CrossRef]
- Brunner, A.; Borate, U.; Esteve, J.; Porkka, K.; Knapper, S.; Vey, N.; Scholl, S.; Wermke, M.; Janssen, J.; Traer, E.; et al. AML-190: Anti-TIM-3 Antibody MBG453 in Combination with Hypomethylating Agents (HMAs) in Patients with High-Risk Myelodysplastic Syndrome (HR-MDS) and Acute Myeloid Leukemia: A Phase 1 Study. Clin. Lymphoma Myeloma Leuk. 2020, 20, S188–S189. [Google Scholar] [CrossRef]
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Traer, E.; Scholl, S.; Garcia-Manero, G.; Vey, N.; Wermke, M.; Janssen, J.; et al. Efficacy and Safety of Sabatolimab (MBG453) in Combination with Hypomethylating Agents (HMAs) in Patients (Pts) with Very High/High-Risk Myelodysplastic Syndrome (vHR/HR-MDS) and Acute Myeloid Leukemia (AML): Final Analysis from a Phase Ib Study. Blood 2021, 138, 244. [Google Scholar] [CrossRef]
- Nolte, M.A.; van Olffen, R.W.; van Gisbergen, K.P.J.M.; van Lier, R.A.W. Timing and tuning of CD27-CD70 interactions: The impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol. Rev. 2009, 229, 216–231. [Google Scholar] [CrossRef]
- Riether, C.; Schürch, C.M.; Bührer, E.D.; Hinterbrandner, M.; Huguenin, A.-L.; Hoepner, S.; Zlobec, I.; Pabst, T.; Radpour, R.; Ochsenbein, A.F. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J. Exp. Med. 2017, 214, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Pabst, T.; Vey, N.; Adès, L.; Bacher, U.; Bargetzi, M.; Fung, S.; Gaidano, G.; Gandini, D.; Hultberg, A.; Johnson, A.; et al. Results from a phase I/II trial of cusatuzumab combined with azacitidine in patients with newly diagnosed acute myeloid leukemia who are ineligible for intensive chemotherapy. Haematologica 2023, 108, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Roboz, G.J.; Pabst, T.; Aribi, A.; Brandwein, J.M.; Döhner, H.; Fiedler, W.; Gandini, D.; Geddes, M.; Hou, J.-Z.; Howes, A.J.; et al. Safety and Efficacy of Cusatuzumab in Combination with Venetoclax and Azacitidine (CVA) in Patients with Previously Untreated Acute Myeloid Leukemia (AML) Who Are Not Eligible for Intensive Chemotherapy; An Open-Label, Multicenter, Phase 1b Study. Blood 2021, 138, 369. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Chen, C.; Liang, C.; Wang, S.; Chio, C.L.; Zhang, Y.; Zeng, C.; Chen, S.; Wang, C.; Li, Y. Expression patterns of immune checkpoints in acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 28. [Google Scholar] [CrossRef]
- Zhong, R.K.; Loken, M.; Lane, T.A.; Ball, E.D. CTLA-4 blockade by a human MAb enhances the capacity of AML-derived DC to induce T-cell responses against AML cells in an autologous culture system. Cytotherapy 2006, 8, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bashey, A.; Medina, B.; Corringham, S.; Pasek, M.; Carrier, E.; Vrooman, L.; Lowy, I.; Solomon, S.R.; Morris, L.E.; Holland, H.K.; et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 2009, 113, 1581–1588. [Google Scholar] [CrossRef]
- Garcia, J.S.; Flamand, Y.; Penter, L.; Keng, M.; Tomlinson, B.K.; Mendez, L.M.; Koller, P.; Cullen, N.; Arihara, Y.; Pfaff, K.; et al. Ipilimumab plus decitabine for patients with MDS or AML in posttransplant or transplant-naïve settings. Blood 2023, 141, 1884–1888. [Google Scholar] [CrossRef] [PubMed]
- Kotwica-Mojzych, K.; Jodłowska-Jędrych, B.; Mojzych, M. CD200:CD200R Interactions and Their Importance in Immunoregulation. Int. J. Mol. Sci. 2021, 22, 1602. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.J.; Wang, E.C.Y.; Man, S.; Hills, R.K.; Burnett, A.K.; Tonks, A.; Darley, R.L. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia 2011, 25, 792–799. [Google Scholar] [CrossRef]
- Memarian, A.; Nourizadeh, M.; Masoumi, F.; Tabrizi, M.; Emami, A.H.; Alimoghaddam, K.; Hadjati, J.; Mirahmadian, M.; Jeddi-Tehrani, M. Upregulation of CD200 is associated with Foxp3+ regulatory T cell expansion and disease progression in acute myeloid leukemia. Tumour Biol. 2013, 34, 531–542. [Google Scholar] [CrossRef]
- Tiribelli, M.; Geromin, A.; Cavallin, M.; Di Giusto, S.; Simeone, E.; Fanin, R.; Damiani, D. ABCG2 and CD200 define patients at high risk of relapse in ELN favorable subgroup of AML. Eur. J. Haematol. 2017, 99, 269–274. [Google Scholar] [CrossRef]
- Tiribelli, M.; Raspadori, D.; Geromin, A.; Cavallin, M.; Sirianni, S.; Simeone, E.; Bocchia, M.; Fanin, R.; Damiani, D. High CD200 expression is associated with poor prognosis in cytogenetically normal acute myeloid leukemia, even in FlT3-ITD-/NPM1+ patients. Leuk. Res. 2017, 58, 31–38. [Google Scholar] [CrossRef]
- Aref, S.; Abousamra, N.; El-Helaly, E.; Mabed, M. Clinical Significance of CD200 and CD56 Expression in Patients with Acute Myeloid Leukemia. Asian Pac. J. Cancer Prev. 2020, 21, 743–748. [Google Scholar] [CrossRef]
- Herbrich, S.; Baran, N.; Cai, T.; Weng, C.; Aitken, M.J.L.; Post, S.M.; Henderson, J.; Shi, C.; Richard-Carpentier, G.; Sauvageau, G.; et al. Overexpression of CD200 is a Stem Cell-Specific Mechanism of Immune Evasion in AML. J. Immunother. Cancer 2021, 9, e002968. [Google Scholar] [CrossRef]
- Rastogi, N.; Baker, S.; Man, S.; Uger, R.A.; Wong, M.; Coles, S.J.; Hodges, M.; Gilkes, A.F.; Knapper, S.; Darley, R.L.; et al. Use of an anti-CD200-blocking antibody improves immune responses to AML in vitro and in vivo. Br. J. Haematol. 2021, 193, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.-M.; Okazaki, T. LAG-3: From molecular functions to clinical applications. J. Immunother. Cancer 2020, 8, e001014. [Google Scholar] [CrossRef]
- Radwan, S.M.; Elleboudy, N.S.; Nabih, N.A.; Kamal, A.M. The immune checkpoints Cytotoxic T lymphocyte antigen-4 and Lymphocyte activation gene-3 expression is up-regulated in acute myeloid leukemia. HLA 2020, 96, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tan, J.; Huang, S.; Huang, X.; Huang, J.; Chen, J.; Yu, Z.; Lu, Y.; Weng, J.; Du, X.; et al. Higher frequency of the CTLA-4(+) LAG-3(+) T-cell subset in patients with newly diagnosed acute myeloid leukemia. Asia. Pac. J. Clin. Oncol. 2020, 16, e12–e18. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, S.; Pievani, A.; Biondi, A.; Dotti, G.; Serafini, M. Catch me if you can: How AML and its niche escape immunotherapy. Leukemia 2022, 36, 13–22. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarnera, L.; Bravo-Perez, C.; Visconte, V. Immunotherapy in Acute Myeloid Leukemia: A Literature Review of Emerging Strategies. Bioengineering 2023, 10, 1228. https://doi.org/10.3390/bioengineering10101228
Guarnera L, Bravo-Perez C, Visconte V. Immunotherapy in Acute Myeloid Leukemia: A Literature Review of Emerging Strategies. Bioengineering. 2023; 10(10):1228. https://doi.org/10.3390/bioengineering10101228
Chicago/Turabian StyleGuarnera, Luca, Carlos Bravo-Perez, and Valeria Visconte. 2023. "Immunotherapy in Acute Myeloid Leukemia: A Literature Review of Emerging Strategies" Bioengineering 10, no. 10: 1228. https://doi.org/10.3390/bioengineering10101228