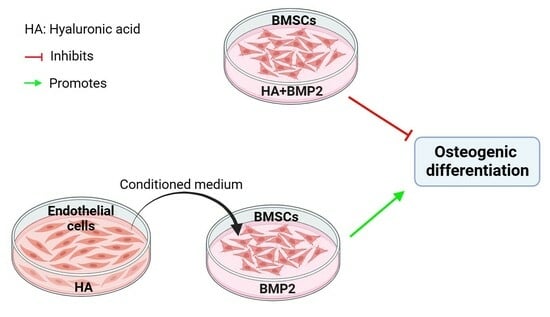
The Paracrine Effect of Hyaluronic Acid-Treated Endothelial Cells Promotes BMP-2-Mediated Osteogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Chemicals
2.2. In Vitro Tube Formation Assay
2.3. Angiogenic Differentiation of HUVECs
2.4. HUVEC Conditioned Medium Preparation and BMSC Culture
2.5. Osteogenic Differentiation Assay
2.5.1. Alkaline Phosphatase (ALP) Staining and ALP Activity Assay
2.5.2. Alizarin Red Staining
2.5.3. mRNA Expression of Osteogenic Markers
2.6. RNA Isolation and Real-Time Quantitative PCR (RT-qPCR) Analysis
2.7. Animals, Anesthesia, and Surgery
2.8. Micro-CT Analysis
2.9. Masson’s Trichrome Staining
2.10. Statistical Analysis
3. Results
3.1. HA Promotes the Angiogenic Differentiation of HUVECs
3.2. HA Inhibits, but CM from HA-Treated HUVECs Promotes, BMP-2-Mediated Osteogenic Differentiation of BMSCs
3.3. HA + BMP-2 Treatment Upregulates Angiogenesis-Related Marker Genes’ Expression in HUVECs
3.4. HA + BMP-2 Synergistically Enhanced In Vivo Bone Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.Y.; Lieu, S.; Lu, C.; Colnot, C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone 2010, 47, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112466. [Google Scholar] [CrossRef] [PubMed]
- Abel, F.; Tan, E.T.; Sneag, D.B.; Lebl, D.R.; Chazen, J.L. Postoperative Lumbar Fusion Bone Morphogenic Protein-Related Epidural Cyst Formation. AJNR Am. J. Neuroradiol. 2023, 44, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhou, T.; Chen, H.; Li, C.; Jiang, Z.; Lao, L.; Kahn, S.A.; Duarte, M.E.L.; Zhao, J.; Daubs, M.D.; et al. Bone morphogenetic protein-2 promotes osteosarcoma growth by promoting epithelial-mesenchymal transition (EMT) through the Wnt/β-catenin signaling pathway. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.Z.; Merrill, R.K.; Kim, J.S.; Overley, S.C.; Dowdell, J.E.; Somani, S.; Hecht, A.C.; Cho, S.K.; Qureshi, S.A. Bone morphogenetic protein use in spine surgery in the United States: How have we responded to the warnings? Spine J. Off. J. N. Am. Spine Soc. 2017, 17, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liao, S.; Li, Y.; Jiang, H.; Liu, Y.; Wang, C.; Kuek, V.; Kenny, J.; Li, B.; Huang, Q.; et al. Osteoblast-derived EGFL6 couples angiogenesis to osteogenesis during bone repair. Theranostics 2021, 11, 9738–9751. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef]
- Schlickewei, C.W.; Kleinertz, H.; Thiesen, D.M.; Mader, K.; Priemel, M.; Frosch, K.-H.; Keller, J. Current and Future Concepts for the Treatment of Impaired Fracture Healing. Int. J. Mol. Sci. 2019, 20, 5805. [Google Scholar] [CrossRef]
- Kaigler, D.; Krebsbach, P.H.; Wang, Z.; West, E.R.; Horger, K.; Mooney, D.J. Transplanted endothelial cells enhance orthotopic bone regeneration. J. Dent. Res. 2006, 85, 633–637. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Agrali, O.B.; Yildirim, S.; Ozener, H.O.; Köse, K.N.; Ozbeyli, D.; Soluk-Tekkesin, M.; Kuru, L. Evaluation of the Effectiveness of Esterified Hyaluronic Acid Fibers on Bone Regeneration in Rat Calvarial Defects. BioMed Res. Int. 2018, 2018, 3874131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-T.; Liu, R.-M.; Luo, Y.; Zhao, Y.-J.; Chen, D.-X.; Yu, C.-Y.; Xiao, J.-H. Hyaluronic acid promotes osteogenic differentiation of human amniotic mesenchymal stem cells via the TGF-β/Smad signalling pathway. Life Sci. 2019, 232, 116669. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Higuchi, C.; Kunugiza, Y.; Yoshida, K.; Sakai, T.; Yoshikawa, H.; Nakata, K. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015, 589, 447–454. [Google Scholar] [CrossRef]
- Kawano, M.; Ariyoshi, W.; Iwanaga, K.; Okinaga, T.; Habu, M.; Yoshioka, I.; Tominaga, K.; Nishihara, T. Mechanism involved in enhancement of osteoblast differentiation by hyaluronic acid. Biochem. Biophys. Res. Commun. 2011, 405, 575–580. [Google Scholar] [CrossRef]
- Huang, H.; Feng, J.; Wismeijer, D.; Wu, G.; Hunziker, E.B. Hyaluronic Acid Promotes the Osteogenesis of BMP-2 in an Absorbable Collagen Sponge. Polymers 2017, 9, 339. [Google Scholar] [CrossRef]
- Shi, W.; Fang, F.; Kong, Y.; Greer, S.E.; Kuss, M.; Liu, B.; Xue, W.; Jiang, X.; Lovell, P.; Mohs, A.M.; et al. Dynamic hyaluronic acid hydrogel with covalent linked gelatin as an anti-oxidative bioink for cartilage tissue engineering. Biofabrication 2021, 14, 014107. [Google Scholar] [CrossRef]
- Mahsa Khatami, S.; Parivar, K.; Naderi Sohi, A.; Soleimani, M.; Hanaee-Ahvaz, H. Acetylated hyaluronic acid effectively enhances chondrogenic differentiation of mesenchymal stem cells seeded on electrospun PCL scaffolds. Tissue Cell 2020, 65, 101363. [Google Scholar] [CrossRef]
- Huang, H.; Wismeijer, D.; Hunziker, E.B.; Wu, G. The Acute Inflammatory Response to Absorbed Collagen Sponge Is Not Enhanced by BMP-2. Int. J. Mol. Sci. 2017, 18, 498. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.; Li, Y.; Zou, J.; Han, R.; Li, H.; Zhang, J. Effect of Puerarin on Osteogenic Differentiation in vitro and on New Bone Formation in vivo. Drug Des. Devel Ther. 2022, 16, 2885–2900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.; She, H.; Wang, R.; Bai, F.; Xiang, B. A silk fibroin/chitosan/nanohydroxyapatite biomimetic bone scaffold combined with autologous concentrated growth factor promotes the proliferation and osteogenic differentiation of BMSCs and repair of critical bone defects. Regen. Ther. 2022, 21, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Kushioka, J.; Chow, S.K.-H.; Toya, M.; Tsubosaka, M.; Shen, H.; Gao, Q.; Li, X.; Zhang, N.; Goodman, S.B. Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy. Inflamm. Regen. 2023, 43, 29. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Brandi, M.L.; Collin-Osdoby, P. Vascular biology and the skeleton. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2006, 21, 183–192. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, W.; Tan, W.; Wu, X.; Dai, Z.; Li, Z.; Yan, Z.; Ji, Y.; Wang, Y.; Su, W.; et al. Injectable MMP1-sensitive microspheres with spatiotemporally controlled exosome release promote neovascularized bone healing. Acta Biomater. 2023, 157, 321–336. [Google Scholar] [CrossRef]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Wang, F.; Qian, H.; Kong, L.; Wang, W.; Wang, X.; Xu, Z.; Chai, Y.; Xu, J.; Kang, Q. Accelerated Bone Regeneration by Astragaloside IV through Stimulating the Coupling of Osteogenesis and Angiogenesis. Int. J. Biol. Sci. 2021, 17, 1821–1836. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Savani, R.C.; Cao, G.; Pooler, P.M.; Zaman, A.; Zhou, Z.; DeLisser, H.M. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J. Biol. Chem. 2001, 276, 36770–36778. [Google Scholar] [CrossRef]
- Park, D.; Kim, Y.; Kim, H.; Kim, K.; Lee, Y.S.; Choe, J.; Hahn, J.H.; Lee, H.; Jeon, J.; Choi, C.; et al. Hyaluronic acid promotes angiogenesis by inducing RHAMM-TGFbeta receptor interaction via CD44-PKCdelta. Mol. Cells 2012, 33, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Sansanaphongpricha, K.; Sonthithai, P.; Kaewkong, P.; Thavornyutikarn, B.; Bamrungsap, S.; Kosorn, W.; Thinbanmai, T.; Saengkrit, N. Hyaluronic acid-coated gold nanorods enhancing BMP-2 peptide delivery for chondrogenesis. Nanotechnology 2020, 31, 435101. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.D.; Abueva, C.; Kim, B.; Lee, B.T. Chitosan-hyaluronic acid polyelectrolyte complex scaffold crosslinked with genipin for immobilization and controlled release of BMP-2. Carbohydr. Polym. 2015, 115, 160–169. [Google Scholar] [CrossRef]
- Kim, J.-J.; Ben Amara, H.; Park, J.-C.; Kim, S.; Kim, T.-I.; Seol, Y.-J.; Lee, Y.-M.; Ku, Y.; Rhyu, I.-C.; Koo, K.-T. Biomodification of compromised extraction sockets using hyaluronic acid and rhBMP-2: An experimental study in dogs. J. Periodontol. 2019, 90, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.; Lunger, A.; Burger, M.G.; Briquez, P.S.; Mai, F.; Hubbell, J.A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. VEGF dose controls the coupling of angiogenesis and osteogenesis in engineered bone. NPJ Regen. Med. 2023, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Liang, Q.; Du, L.; Shang, L.; Wang, T.; Ge, S. Sequential application of bFGF and BMP-2 facilitates osteogenic differentiation of human periodontal ligament stem cells. J. Periodontal Res. 2019, 54, 424–434. [Google Scholar] [CrossRef]
- Qi, W.; Yan, J.; Sun, H.; Wang, H. Multifunctional Nanocomposite Films for Synergistic Delivery of bFGF and BMP-2. ACS Omega 2017, 2, 899–909. [Google Scholar] [CrossRef]








| Gene | Primer Sequence | Product Length (bp) |
|---|---|---|
| Mus GAPDH | F: GTGAAGGTCGGTGTGAACGG | 227 |
| R: TCCTGGAAGATGGTGATGGG | ||
| Mus Collagen I | F: ATGCCGCGACCTCAAGATG | 140 |
| R: TGAGGCACAGACGGCTGAGTA | ||
| Mus RUNX2 | F: TGAGGCACAGACGGCTGAGTA | 126 |
| R: CACTGGCGGTGCAACAAGA | ||
| Mus OPN | F: ACCATGCAGAGAGCGAGGATT | 91 |
| R: GGGACATCGACTGTAGGGACG | ||
| Mus ALP | F: TGCCTACTTGTGTGGCGTGAA | 159 |
| R: TCACCCGAGTGGTAGTCAVAATG | ||
| Homo GAPDH | F: GAAGGTGAAGGTCGGAGTCA | 172 |
| R: GAAGATGGTGATGGGATTTC | ||
| Homo CD31 | F: CTCCAGACTCCACCACCTTAC | 243 |
| R: GAACTTTGCCTATTTCTTACCA | ||
| Homo VEGF | F: GGAGGCAGAGAAAAGAGAAAGTGT | 175 |
| R: TAAGAGAGCAAGAGAGAGCAAAAGA | ||
| Homo bFGF | F: AGCCAGGTAACGGTTAGCACA | 91 |
| R: GAAGAGCGACCCTCACATCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Chen, J.; Wang, R.; Hou, D.; Wu, G.; Liu, C.; Pathak, J.L. The Paracrine Effect of Hyaluronic Acid-Treated Endothelial Cells Promotes BMP-2-Mediated Osteogenesis. Bioengineering 2023, 10, 1227. https://doi.org/10.3390/bioengineering10101227
Tong X, Chen J, Wang R, Hou D, Wu G, Liu C, Pathak JL. The Paracrine Effect of Hyaluronic Acid-Treated Endothelial Cells Promotes BMP-2-Mediated Osteogenesis. Bioengineering. 2023; 10(10):1227. https://doi.org/10.3390/bioengineering10101227
Chicago/Turabian StyleTong, Xiaojie, Jin Chen, Renqin Wang, Dan Hou, Gang Wu, Chang Liu, and Janak Lal Pathak. 2023. "The Paracrine Effect of Hyaluronic Acid-Treated Endothelial Cells Promotes BMP-2-Mediated Osteogenesis" Bioengineering 10, no. 10: 1227. https://doi.org/10.3390/bioengineering10101227