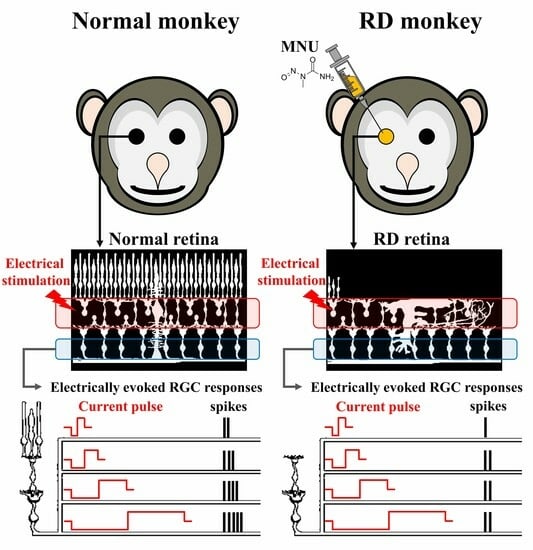
The Variation of Electrical Pulse Duration Elicits Reliable Network-Mediated Responses of Retinal Ganglion Cells in Normal, Not in Degenerate Primate Retinas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Infrared (IR) and Spectral-Domain Optical Coherence Tomography (OCT) Image Acquisition and Analysis
2.3. Retinal Preparation and In Vitro Recording
2.4. Data Analysis
3. Results
3.1. MNU Induces Rod Photoreceptor-Specific Retinal Degeneration in Monkey Retinas
3.2. RGCs in RD Monkey Retinas Show Imprecise Spike Response Time with Repeated Electrical Stimulation
3.3. RD Monkey Retinas Need Higher Threshold Charge Density Thane Normal Monkey Retinas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, F.; Novelli, E.; Strettoi, E. Retinal ganglion cells survive and maintain normal dendritic morphology in a mouse model of inherited photoreceptor degeneration. J. Neurosci. 2008, 28, 14282–14292. [Google Scholar] [CrossRef] [PubMed]
- Margalit, E.; Maia, M.; Weiland, J.D.; Greenberg, R.J.; Fujii, G.Y.; Torres, G.; Piyathaisere, D.V.; O’Hearn, T.M.; Liu, W.; Lazzi, G.; et al. Retinal prosthesis for the blind. Surv. Ophthalmol. 2002, 47, 335–356. [Google Scholar] [CrossRef]
- Stingl, K.; Schippert, R.; Bartz-Schmidt, K.U.; Besch, D.; Cottriall, C.L.; Edwards, T.L.; Gekeler, F.; Greppmaier, U.; Kiel, K.; Koitschev, A.; et al. Interim Results of a Multicenter Trial with the New Electronic Subretinal Implant Alpha AMS in 15 Patients Blind from Inherited Retinal Degenerations. Front. Neurosci. 2017, 11, 445. [Google Scholar] [CrossRef]
- Ayton, L.N.; Barnes, N.; Dagnelie, G.; Fujikado, T.; Goetz, G.; Hornig, R.; Jones, B.W.; Muqit, M.M.K.; Rathbun, D.L.; Stingl, K.; et al. An update on retinal prostheses. Clin. Neurophysiol. 2020, 131, 1383–1398. [Google Scholar] [CrossRef] [PubMed]
- Im, M.; Kim, S.W. Neurophysiological and medical considerations for better-performing microelectronic retinal prostheses. J. Neural Eng. 2020, 17, 033001. [Google Scholar] [CrossRef]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Strettoi, E. Neural remodeling in retinal degeneration. Prog. Retin. Eye Res. 2003, 22, 607–655. [Google Scholar] [CrossRef]
- Biswas, S.; Haselier, C.; Mataruga, A.; Thumann, G.; Walter, P.; Muller, F. Pharmacological analysis of intrinsic neuronal oscillations in rd10 retina. PLoS ONE 2014, 9, e99075. [Google Scholar] [CrossRef]
- Ahn, J.; Cha, S.; Choi, K.E.; Kim, S.W.; Yoo, Y.; Goo, Y.S. Correlated Activity in the Degenerate Retina Inhibits Focal Response to Electrical Stimulation. Front. Cell. Neurosci. 2022, 16, 889663. [Google Scholar] [CrossRef]
- Cha, S.; Ahn, J.; Jeong, Y.; Lee, Y.H.; Kim, H.K.; Lee, D.; Yoo, Y.; Goo, Y.S. Stage-Dependent Changes of Visual Function and Electrical Response of the Retina in the rd10 Mouse Model. Front. Cell. Neurosci. 2022, 16, 926096. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Lee, J.I.; Jang, Y.J.; An, S.; Kim, J.H.; Fried, S.I.; Im, M. Retinal Degeneration Reduces Consistency of Network-Mediated Responses Arising in Ganglion Cells to Electric Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.L.; Jobling, A.I.; Vessey, K.A.; Luu, C.; Guymer, R.H.; Baird, P.N. Animal models of retinal disease. Prog. Mol. Biol. Transl. Sci. 2011, 100, 211–286. [Google Scholar] [CrossRef] [PubMed]
- Winkler, P.A.; Occelli, L.M.; Petersen-Jones, S.M. Large Animal Models of Inherited Retinal Degenerations: A Review. Cells 2020, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Grunert, U.; Martin, P.R. Cell types and cell circuits in human and non-human primate retina. Prog. Retin. Eye Res. 2020, 78, 100844. [Google Scholar] [CrossRef]
- Kostic, C.; Arsenijevic, Y. Animal modelling for inherited central vision loss. J. Pathol. 2016, 238, 300–310. [Google Scholar] [CrossRef]
- Picaud, S.; Dalkara, D.; Marazova, K.; Goureau, O.; Roska, B.; Sahel, J.A. The primate model for understanding and restoring vision. Proc. Natl. Acad. Sci. USA 2019, 116, 26280–26287. [Google Scholar] [CrossRef]
- Moshiri, A.; Chen, R.; Kim, S.; Harris, R.A.; Li, Y.; Raveendran, M.; Davis, S.; Liang, Q.; Pomerantz, O.; Wang, J.; et al. A nonhuman primate model of inherited retinal disease. J. Clin. Investig. 2019, 129, 863–874. [Google Scholar] [CrossRef]
- Peterson, S.M.; McGill, T.J.; Puthussery, T.; Stoddard, J.; Renner, L.; Lewis, A.D.; Colgin, L.M.A.; Gayet, J.; Wang, X.; Prongay, K.; et al. Bardet-Biedl Syndrome in rhesus macaques: A nonhuman primate model of retinitis pigmentosa. Exp. Eye Res. 2019, 189, 107825. [Google Scholar] [CrossRef]
- Choi, K.E.; Cha, S.; Yun, C.; Ahn, J.; Hwang, S.; Kim, Y.J.; Jung, H.; Eom, H.; Shin, D.; Oh, J.; et al. Outer retinal degeneration in a non-human primate model using temporary intravitreal tamponade with N-methyl-N-nitrosourea in cynomolgus monkeys. J. Neural Eng. 2023, 20, 015001. [Google Scholar] [CrossRef]
- Boinagrov, D.; Pangratz-Fuehrer, S.; Goetz, G.; Palanker, D. Selectivity of direct and network-mediated stimulation of the retinal ganglion cells with epi-, sub- and intraretinal electrodes. J. Neural Eng. 2014, 11, 026008. [Google Scholar] [CrossRef]
- Fried, S.I.; Hsueh, H.A.; Werblin, F.S. A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation. J. Neurophysiol. 2006, 95, 970–978. [Google Scholar] [CrossRef]
- Sekirnjak, C.; Hottowy, P.; Sher, A.; Dabrowski, W.; Litke, A.M.; Chichilnisky, E.J. Electrical stimulation of mammalian retinal ganglion cells with multielectrode arrays. J. Neurophysiol. 2006, 95, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Jepson, L.H.; Hottowy, P.; Mathieson, K.; Gunning, D.E.; Dabrowski, W.; Litke, A.M.; Chichilnisky, E.J. Focal electrical stimulation of major ganglion cell types in the primate retina for the design of visual prostheses. J. Neurosci. 2013, 33, 7194–7205. [Google Scholar] [CrossRef] [PubMed]
- Yucel, E.I.; Sadeghi, R.; Kartha, A.; Montezuma, S.R.; Dagnelie, G.; Rokem, A.; Boynton, G.M.; Fine, I.; Beyeler, M. Factors affecting two-point discrimination in Argus II patients. Front. Neurosci. 2022, 16, 901337. [Google Scholar] [CrossRef] [PubMed]
- Madugula, S.S.; Gogliettino, A.R.; Zaidi, M.; Aggarwal, G.; Kling, A.; Shah, N.P.; Brown, J.B.; Vilkhu, R.; Hays, M.R.; Nguyen, H.; et al. Focal electrical stimulation of human retinal ganglion cells for vision restoration. J. Neural Eng. 2022, 19, 066040. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.; Morley, J.W.; Suaning, G.J.; Lovell, N.H. Direct activation and temporal response properties of rabbit retinal ganglion cells following subretinal stimulation. J. Neurophysiol. 2009, 102, 2982–2993. [Google Scholar] [CrossRef]
- Web Vision the Architecture of the Human Fovea. Available online: https://webvision.med.utah.edu/book/part-ii-anatomy-and-physiology-of-the-retina/the-architecture-of-the-human-fovea/ (accessed on 20 September 2023).
- Ahn, J.; Jeong, Y.; Cha, S.; Lee, J.Y.; Yoo, Y.; Goo, Y.S. High amplitude pulses on the same charge condition efficiently elicit bipolar cell-mediated retinal ganglion cell responses in the degenerate retina. Biomed. Eng. Lett. 2023, 13, 129–140. [Google Scholar] [CrossRef]
- Ahn, J.; Choi, M.H.; Kim, K.; Senok, S.S.; Cho, D.D.; Koo, K.I.; Goo, Y. The advantage of topographic prominence-adopted filter for the detection of short-latency spikes of retinal ganglion cells. Korean J. Physiol. Pharmacol. 2017, 21, 555–563. [Google Scholar] [CrossRef]
- Nagar, S.; Krishnamoorthy, V.; Cherukuri, P.; Jain, V.; Dhingra, N.K. Early remodeling in an inducible animal model of retinal degeneration. Neuroscience 2009, 160, 517–529. [Google Scholar] [CrossRef]
- Reisenhofer, M.; Pannicke, T.; Reichenbach, A.; Enzmann, V. Characteristics of Muller glial cells in MNU-induced retinal degeneration. Vis. Neurosci. 2016, 33, E013. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Omi, N. OFF bipolar cells in macaque retina: Type-specific connectivity in the outer and inner synaptic layers. Front. Neuroanat. 2015, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Omi, N. ON Bipolar Cells in Macaque Retina: Type-Specific Synaptic Connectivity with Special Reference to OFF Counterparts. Front. Neuroanat. 2016, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Omi, N. Classification of Mouse Retinal Bipolar Cells: Type-Specific Connectivity with Special Reference to Rod-Driven AII Amacrine Pathways. Front. Neuroanat. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.; Otgondemberel, Y.; Im, M. Short pulses of epiretinal prostheses evoke network-mediated responses in retinal ganglion cells by stimulating presynaptic neurons. J. Neural Eng. 2022, 19, 055006. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Choi, K.E.; Ahn, J.; Yoo, M.; Jeong, Y.; Kim, S.W.; Goo, Y.S. Electrical response of retinal ganglion cells in an N-methyl-N-nitrosourea-induced retinal degeneration porcine model. Sci. Rep. 2021, 11, 24135. [Google Scholar] [CrossRef] [PubMed]
- Gargini, C.; Terzibasi, E.; Mazzoni, F.; Strettoi, E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: A morphological and ERG study. J. Comp. Neurol. 2007, 500, 222–238. [Google Scholar] [CrossRef]
- Kovacs-Oller, T.; Dedek, K.; Hillier, D. Editorial: Visual code: From the retina to the brain. Front. Cell. Neurosci. 2022, 16, 1018229. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, S.; Ahn, J.; Kim, S.-W.; Choi, K.-E.; Yoo, Y.; Eom, H.; Shin, D.; Goo, Y.S. The Variation of Electrical Pulse Duration Elicits Reliable Network-Mediated Responses of Retinal Ganglion Cells in Normal, Not in Degenerate Primate Retinas. Bioengineering 2023, 10, 1135. https://doi.org/10.3390/bioengineering10101135
Cha S, Ahn J, Kim S-W, Choi K-E, Yoo Y, Eom H, Shin D, Goo YS. The Variation of Electrical Pulse Duration Elicits Reliable Network-Mediated Responses of Retinal Ganglion Cells in Normal, Not in Degenerate Primate Retinas. Bioengineering. 2023; 10(10):1135. https://doi.org/10.3390/bioengineering10101135
Chicago/Turabian StyleCha, Seongkwang, Jungryul Ahn, Seong-Woo Kim, Kwang-Eon Choi, Yongseok Yoo, Heejong Eom, Donggwan Shin, and Yong Sook Goo. 2023. "The Variation of Electrical Pulse Duration Elicits Reliable Network-Mediated Responses of Retinal Ganglion Cells in Normal, Not in Degenerate Primate Retinas" Bioengineering 10, no. 10: 1135. https://doi.org/10.3390/bioengineering10101135