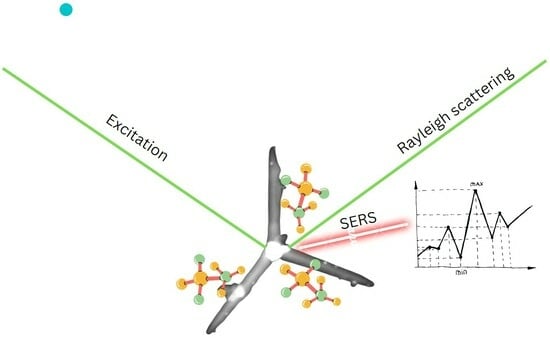
Zinc Oxide Tetrapods Doped with Silver Nanoparticles as a Promising Substrate for the Detection of Biomolecules via Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization of ZnO-Ag Tetrapods
2.3. Fabrication of SERS Substrates
2.4. Characterization of SERS Substrates by SEM
2.5. Preparation of RhB Solution Series
2.6. Raman Measurements
3. Results
3.1. Morphological Studies
3.2. Elemental Composition Analysis: Insights from EDX Measurements
3.3. XRD Analysis
3.4. SERS Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, S.; Cheng, Z.; Li, Q.; Wang, R.; Yu, F. Toward Sensitive and Reliable Surface-Enhanced Raman Scattering Imaging: From Rational Design to Biomedical Applications. ACS Sens. 2021, 6, 3912–3932. [Google Scholar] [CrossRef]
- Szymborski, T.; Stepanenko, Y.; Niciński, K.; Piecyk, P.; Berus, S.M.; Adamczyk-Popławska, M.; Kamińska, A. Ultrasensitive SERS Platform Made via Femtosecond Laser Micromachining for Biomedical Applications. J. Mater. Res. Technol. 2021, 12, 1496–1507. [Google Scholar] [CrossRef]
- Sitjar, J.; Liao, J.-D.; Lee, H.; Tsai, H.-P.; Wang, J.-R.; Liu, P.-Y. Challenges of SERS Technology as a Non-Nucleic Acid or -Antigen Detection Method for SARS-CoV-2 Virus and Its Variants. Biosens. Bioelectron. 2021, 181, 113153. [Google Scholar] [CrossRef]
- Laghrib, F.; Saqrane, S.; El Bouabi, Y.; Farahi, A.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Current Progress on COVID-19 Related to Biosensing Technologies: New Opportunity for Detection and Monitoring of Viruses. Microchem. J. 2021, 160, 105606. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Tena, N.; Asuero, A.G. Current State of Diagnostic, Screening and Surveillance Testing Methods for COVID-19 from an Analytical Chemistry Point of View. Microchem. J. 2021, 167, 106305. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhou, H.S. Diagnostic Methods and Potential Portable Biosensors for Coronavirus Disease 2019. Biosens. Bioelectron. 2020, 165, 112349. [Google Scholar] [CrossRef]
- Asif, M.; Ajmal, M.; Ashraf, G.; Muhammad, N.; Aziz, A.; Iftikhar, T.; Wang, J.; Liu, H. The Role of Biosensors in Coronavirus Disease-2019 Outbreak. Curr. Opin. Electrochem. 2020, 23, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Guo, S.; Jin, S.; Chen, L.; Jung, Y. Biomarkers Determination Based on Surface-Enhanced Raman Scattering. Chemosensors 2020, 8, 118. [Google Scholar] [CrossRef]
- Maddali, H.; Miles, C.E.; Kohn, J.; O’Carroll, D.M. Optical Biosensors for Virus Detection: Prospects for SARS-CoV-2/COVID-19. ChemBioChem 2020, 22, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Ambrosch-Draxl, C.; Auer, H.; Kouba, R.; Sherman, E.Y.; Knoll, P.; Mayer, M. Raman Scattering inYBa2Cu3O7: A Comprehensive Theoretical Study in Comparison with Experiments. Phys. Rev. B 2002, 65, 064501. [Google Scholar] [CrossRef]
- Gillet, Y.; Giantomassi, M.; Gonze, X. First-Principles Study of Excitonic Effects in Raman Intensities. Phys. Rev. B 2013, 88, 094305. [Google Scholar] [CrossRef]
- Mosier-Boss, P. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, Applications, and the Future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Ali, A.; Nettey-Oppong, E.E.; Effah, E.; Yu, C.Y.; Muhammad, R.; Soomro, T.A.; Byun, K.M.; Choi, S.H. Miniaturized Raman Instruments for SERS-Based Point-of-Care Testing on Respiratory Viruses. Biosensors 2022, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, E.; Shi, H.; Tao, Y.; Ren, X. Semiconductor-Based Surface Enhanced Raman Scattering (SERS): From Active Materials to Performance Improvement. Analyst 2022, 147, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Manikandan, P.; Manikandan, D.; Umapathy, S.; Padhy, H.M.; Maaza, M.; Elayaperumal, M. Noble Metal Ion Embedded Nanocomposite Glass Materials for Optical Functionality of UV–Visible Surface Plasmon Resonance (SPR) Surface-Enhanced Raman Scattering (SERS) X-ray and Electron Microscopic Studies: An Overview. Plasmonics 2021, 16, 1461–1493. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Meyer, M.; Blackie, E.; Etchegoin, P.G. Advanced Aspects of Electromagnetic SERS Enhancement Factors at a Hot Spot. J. Raman Spectrosc. 2008, 39, 1127–1134. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.; Kim, N.-J.; Kim, H.; Yi, G.-C.; Shin, Y.; Kim, M.H.; Yoon, S. Study of Chemical Enhancement Mechanism in Non-Plasmonic Surface Enhanced Raman Spectroscopy (SERS). Front. Chem. 2019, 7, 582. [Google Scholar] [CrossRef]
- Trivedi, D.J.; Barrow, B.; Schatz, G.C. Understanding the Chemical Contribution to the Enhancement Mechanism in SERS: Connection with Hammett Parameters. J. Chem. Phys. 2020, 153, 124706. [Google Scholar] [CrossRef]
- Mohaghegh, F.; Tehrani, A.M.; Materny, A. Investigation of the Importance of the Electronic Enhancement Mechanism for Surface-Enhanced Raman Scattering (SERS). J. Phys. Chem. C 2021, 125, 5158–5166. [Google Scholar] [CrossRef]
- Tan, L.; Wei, M.; Shang, L.; Yang, Y. Cucurbiturils-Mediated Noble Metal Nanoparticles for Applications in Sensing, SERS, Theranostics, and Catalysis. Adv. Funct. Mater. 2020, 31, 202007277. [Google Scholar] [CrossRef]
- Trausa, A.; Tipaldi, C.F.; Ignatane, L.; Polyakov, B.; Oras, S.; Butanovs, E.; Vanags, E.; Smits, K. Heat-Induced Fragmentation and Adhesive Behaviour of Gold Nanowires for Surface-Enhanced Raman Scattering Substrates. Chem. Eng. 2024, 8, 15. [Google Scholar] [CrossRef]
- Samriti; Rajput, V.; Gupta, R.K.; Prakash, J. Engineering Metal Oxide Semiconductor Nanostructures for Enhanced Charge Transfer: Fundamentals and Emerging SERS Applications. J. Mater. Chem. C 2022, 10, 73–95. [Google Scholar] [CrossRef]
- Liang, X.; Li, N.; Zhang, R.; Yin, P.; Zhang, C.; Yang, N.; Liang, K.; Kong, B. Carbon-Based SERS Biosensor: From Substrate Design to Sensing and Bioapplication. NPG Asia Mater. 2021, 13, 8. [Google Scholar] [CrossRef]
- Li, D.; Wu, A.; Wan, Q.; Li, Z. Controllable Fabrication of Polymeric Nanowires by NIL Technique and Self-Assembled AAO Template for SERS Application. Sci. Rep. 2021, 11, 14929. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Tardivel, M.; Lequeux, M.; Chen, X.; Liu, W.; Huang, J.; Tian, H.; Liu, Q.; Huang, G.; et al. Evaluation of the Reliability of Six Commercial SERS Substrates. Plasmonics 2019, 15, 743–752. [Google Scholar] [CrossRef]
- Das, G.; Mecarini, F.; Gentile, F.; De Angelis, F.; Mohan Kumar, H.; Candeloro, P.; Liberale, C.; Cuda, G.; Di Fabrizio, E. Nano-Patterned SERS Substrate: Application for Protein Analysis vs. Temperature. Biosens. Bioelectron. 2009, 24, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, H.; Tan, X.; Lu, Z.; Han, H. Cauliflower-Inspired 3D SERS Substrate for Multiple Mycotoxins Detection. Anal. Chem. 2019, 91, 3885–3892. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.-B.; Tian, X.-D.; Song, J.-J.; Wen, B.-Y.; Zhang, Y.-J.; Fang, P.-P.; Li, J.-F. Self-Calibration 3D Hybrid SERS Substrate and Its Application in Quantitative Analysis. Anal. Chem. 2022, 94, 9578–9585. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Riswana Barveen, N.; Wang, T.-J.; Chang, Y.-H. In-Situ Deposition of Silver Nanoparticles on Silver Nanoflowers for Ultrasensitive and Simultaneous SERS Detection of Organic Pollutants. Microchem. J. 2020, 159, 105520. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Jiang, Z.; Shi, Z.; Wang, J.; Du, L. Highly Sensitive and Reproducible SERS Substrates Based on Ordered Micropyramid Array and Silver Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 29222–29229. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Picciolini, S.; Castagnetti, N.; Vanna, R.; Mehn, D.; Bedoni, M.; Gramatica, F.; Villani, M.; Calestani, D.; Pavesi, M.; Lazzarini, L.; et al. Branched Gold Nanoparticles on ZnO 3D Architecture as Biomedical SERS Sensors. RSC Adv. 2015, 5, 93644–93651. [Google Scholar] [CrossRef]
- Xu, J.; Li, C.; Si, H.; Zhao, X.; Wang, L.; Jiang, S.; Wei, D.; Yu, J.; Xiu, X.; Zhang, C. 3D SERS Substrate Based on Au-Ag Bi-Metal Nanoparticles/MoS2 Hybrid with Pyramid Structure. Opt. Express 2018, 26, 21546. [Google Scholar] [CrossRef] [PubMed]
- Pahlow, S.; Mayerhöfer, T.; van der Loh, M.; Hübner, U.; Dellith, J.; Weber, K.; Popp, J. Interference-Enhanced Raman Spectroscopy as a Promising Tool for the Detection of Biomolecules on Raman-Compatible Surfaces. Anal. Chem. 2018, 90, 9025–9032. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Adelung, R. ZnO Tetrapod Materials for Functional Applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]








| Sample | Zinc, Atomic % | Oxygen, Atomic % | Silver, Atomic % |
|---|---|---|---|
| ZnAg0 | 51.1 ± 7.5 | 48.9 ± 7.8 | - |
| ZnAg1 | 51.1 ± 6.0 | 48.7 ± 6.0 | 0.15 ± 0.12 |
| ZnAg5 | 51.7 ± 2.4 | 46.7 ± 1.7 | 1.5 ± 1.4 |
| ZnAg10 | 49.5 ± 5.3 | 48.7 ± 0.2 | 4.1 ± 1.9 |
| ZnXAg0 | 52.5 ± 1.5 | 47.5 ± 1.2 | - |
| ZnXAg1 | 46.5 ±4.3 | 50.4 ± 2.7 | 3.4 ± 2.9 |
| ZnXAg5 | 46.5 ± 6.4 | 47.6 ± 4.7 | 5.9 ± 1.1 |
| ZnXAg10 | 38.3 ± 2.0 | 44.5 ± 2.6 | 17.3 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanags, E.; Bite, I.; Ignatane, L.; Ignatans, R.; Trausa, A.; Tipaldi, C.F.; Vilks, K.; Smits, K. Zinc Oxide Tetrapods Doped with Silver Nanoparticles as a Promising Substrate for the Detection of Biomolecules via Surface-Enhanced Raman Spectroscopy. ChemEngineering 2024, 8, 19. https://doi.org/10.3390/chemengineering8010019
Vanags E, Bite I, Ignatane L, Ignatans R, Trausa A, Tipaldi CF, Vilks K, Smits K. Zinc Oxide Tetrapods Doped with Silver Nanoparticles as a Promising Substrate for the Detection of Biomolecules via Surface-Enhanced Raman Spectroscopy. ChemEngineering. 2024; 8(1):19. https://doi.org/10.3390/chemengineering8010019
Chicago/Turabian StyleVanags, Edgars, Ivita Bite, Liga Ignatane, Reinis Ignatans, Annamarija Trausa, Ciro Federiko Tipaldi, Karlis Vilks, and Krisjanis Smits. 2024. "Zinc Oxide Tetrapods Doped with Silver Nanoparticles as a Promising Substrate for the Detection of Biomolecules via Surface-Enhanced Raman Spectroscopy" ChemEngineering 8, no. 1: 19. https://doi.org/10.3390/chemengineering8010019