Therapeutic Potential of Two Derivative Prescriptions of Rokumijiogan, Hachimijiogan and Bakumijiogan against Renal Damage in Nephrectomized Rats
Abstract
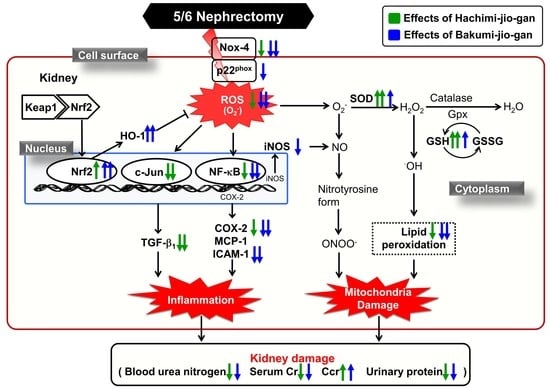
:1. Introduction
2. HJG and BJG Extracts
3. Renal Histological Findings and Renal Functional Parameters
4. Biomarkers Associated with Oxidative Stress in the Kidney
5. Renal NADPH Oxidase-4 (Nox-4), p22phox, Nrf2, and Heme Oxygenase-1 (HO-1) Protein Expressions
6. Renal c-Jun N-Terminal Kinase (JNK), Phosphor (p)-JNK, c-Jun, and Transforming Growth Factor-β1 (TGF-β1) Protein Expressions
7. Renal NF-κB, Cyclooxygenase-2 (COX-2), Inducible Nitric Oxide Synthase (iNOS), Monocyte Chemotactic Protein-1 (MCP-1), and ICAM-1 Protein Expressions
8. Protective Role of RJG-Containing Components against Free Radical-Induced Oxidative Stress in Renal Tubular Epithelial Cells
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Radhakrishnan, J.; Remuzzim, G.; Saran, R.; Williams, D.E.; Rios-Burrows, N.; Powe, N.; Brück, K.; Wanner, C.; Stel, V.; Venuthurupalli, S.; et al. Taming the chronic kidney disease epidemic: A global view of surveillance efforts. Kidney Int. 2014, 86, 246–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, Z.S.; Griva, K. Anxiety and depression in patients with end-stage renal disease: Impact and management challenges—A narrative review. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astin, J.A.; Marie, A.; Pelletier, K.R.; Hansen, E.; Haskell, W.L. A review of the incorporation of complementary and alternative medicine by mainstream physicians. Arch. Intern. Med. 1998, 158, 2303–2310. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.; Lee, J.K.; Lee, H.Y.; Koh, W.S.; Seo, C.S.; Lee, M.Y.; Huang, D.S.; Shin, H. Safety evaluation of Yukmijihwang-tang: Assessment of acute and subchronic toxicity in rats. Evid. Based Complement. Alternat. Med. 2011, 2011, 672136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singla, R.K.; De, R.; Efferth, T.; Mezzetti, B.; Sahab Uddin, M.; Sanusi; Ntie-Kang, F.; Wang, D.; Schultz, F.; Kharat, K.R.; et al. The International Natural Product Sciences Taskforce (INPST) and the power of Twitter networking exemplified through # INPST hashtag analysis. Phytomed. Int. J. Phytother. Phytopharm. 2023, 108, 154520. [Google Scholar] [CrossRef]
- Lu, Z.; Zhong, Y.; Liu, W.; Xiang, L.; Deng, Y. The efficacy and mechanism of Chinese herbal medicine on diabetic kidney disease. J. Diabetes Res. 2019, 2019, 2697672. [Google Scholar] [CrossRef]
- Kang, D.G.; Sohn, E.J.; Moon, M.K.; Mun, Y.J.; Woo, W.H.; Kim, M.K.; Lee, H.S. Yukmijihwang-tang ameliorates ischemia/reperfusion-induced renal injury in rats. J. Ethnopharmacol. 2006, 104, 47–53. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, N.H.; Ha, H.K.; Lee, H.Y.; Jung, D.Y.; Choi, J.Y.; Seo, C.S.; Shin, H.K. Analysis of studies on Yukmijihwang-tang for establishment of evidence based medicine. Korean J. Orient. Physiol. Pathol. 2009, 23, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Kondo, T. Kidneys in oriental and occidental medicine. Integr. Med. Int. 2016, 3, 64–67. [Google Scholar] [CrossRef]
- Liu, Y.L.; Lee, W.C. Traditional Chinese medicine and herbal supplements for treating overactive bladder. Urol. Sci. 2018, 29, 216–222. [Google Scholar] [CrossRef]
- Shimizu, M.; Takayama, S.; Ishizawa, K.; Abe, M.; Ishii, T. Kampo medicine can improve quality of life and prolong hemodialysis implementation in patients with advanced-stage chronic kidney disease. Trad. Kampo Med. 2021, 8, 229–233. [Google Scholar] [CrossRef]
- Lv, W.; Booz, G.W.; Fan, F.; Wang, Y.; Roman, R.L. Oxidative stress and renal fibrosis: Recent insights for the development of novel therapeutic strategies. Front. Physiol. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Lee, S.L.; Noh, J.S.; Yokozawa, T. Rokumi-jio-gan-containing prescriptions attenuate oxidative stress, inflammation, and apoptosis in the remnant kidney. Evid. Based Complement. Alternat. Med. 2012, 2012, 587902. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, S.; Okamoto, T.; Tanaka, T.; Yokozawa, T. Rokumi-jio-gan-containing prescriptions regulate oxidative stress through improving dyslipidemia in a subtotal nephrectomized rat model. J. Ethnopharmacol. 2013, 148, 449–458. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Bernard, D.B.; Donohoe, J.F.; Levinsky, N.G. Ischemic damage and repair in the rat proximal tubule: Differences among the S1, S2, and S3 segments. Kidney Int. 1978, 14, 31–49. [Google Scholar] [CrossRef] [Green Version]
- Paller, M.S.; Neumann, T.V. Reactive oxygen species and rat renal epithelial cells during hypoxia and reoxygenation. Kidney Int. 1991, 40, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Remuzzi, G.; Ruggenenti, P.; Benigni, A. Understanding the nature of renal disease progression. Kidney Int. 1997, 51, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Remuzzi, G.; Bertani, T. Pathophysiology of progressive nephropathies. N. Engl. J. Med. 1998, 339, 1448–1456. [Google Scholar] [CrossRef]
- Brenner, B.M.; Meyer, T.W.; Hostetter, T.H. Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 1982, 307, 652–659. [Google Scholar] [CrossRef]
- Sakai, T.; Kumano, K.; Takara, S.; Kuwao, S.; Ise, M.; Sugano, M.; Uehara, Y. Effect of oral-adsorbent (AST-120) to chronic renal failure (CRF) in rats. Jpn. J. Nephrol. 1989, 31, 359–365. [Google Scholar] [CrossRef]
- Harris, D.C.; Chan, L.; Schrier, R.W. Remnant kidney hypermetabolism and progression of chronic renal failure. Am. J. Physiol. Ren. Physiol. 1988, 254, F267–F276. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012, 2012, 465897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamay-Cach, F.; Quintana-Pérez, J.C.; Trujillo-Ferrara, J.G.; Cuevas-Hernández, R.I.; Del Valle-Mondragón, L.; García-Trejo, E.M.; Arellano-Mendoza, M.G. A review of the impact of oxidative stress and some antioxidant therapies on renal damage. Ren. Fail. 2016, 38, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Ling, X.C.; Kuo, K.L. Oxidative stress in chronic kidney disease. Ren. Replace Ther. 2018, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Vaziri, N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Ren. Physiol. 2010, 298, F662–F671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Lambeth, J.D.; Nauseef, W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002, 397, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Etoh, T.; Inoguchi, T.; Kakimoto, M.; Sonoda, N.; Kobayashi, K.; Kuroda, J.; Sumimoto, H.; Nawata, H. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia 2003, 46, 1428–1437. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, T.; Ritsick, D.; Cheng, G.; Lambeth, J.D. Point mutation in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J. Biol. Chem. 2005, 280, 31859–31869. [Google Scholar] [CrossRef] [Green Version]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [Green Version]
- Tong, K.I.; Katoh, Y.; Kusunoki, H.; Itoh, K.; Tanaka, T.; Yamamoto, M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol. Cell. Biol. 2006, 26, 2887–2900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, C.J.; Thimmulappa, R.K.; Singh, A.; Blake, D.J.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, A.A.; Li, F.Y.L.; Leake, D.S.; Ishii, T.; Mann, G.E.; Siow, R.C.M. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: Role of mitogen-activated protein kinases and Nrf2. Free Radic. Biol. Med. 2005, 39, 227–236. [Google Scholar] [CrossRef]
- He, M.; Siow, R.C.M.; Sugden, D.; Gao, L.; Cheng, X.; Mann, G.E. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: A role for Nrf2 in vascular protection in diabetes. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 277–285. [Google Scholar] [CrossRef]
- Hojo, Y.; Saito, Y.; Tanimoto, T.; Hoefen, R.J.; Baines, C.P.; Yamamoto, K.; Haendeler, J.; Asmis, R.; Berk, B.C. Fluid shear stress attenuates hydrogen peroxide-induced c-Jun NH2-terminal kinase activation via a glutathione reductase-mediated mechanism. Circ. Res. 2002, 91, 712–718. [Google Scholar] [CrossRef] [Green Version]
- Kallunki, T.; Deng, T.; Hibi, M.; Karin, M. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell 1996, 87, 929–939. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Campbell, D.; Dériijard, B.; Davis, R.J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 1995, 267, 389–393. [Google Scholar] [CrossRef]
- Hocevar, B.A.; Brown, T.L.; Howe, P.H. TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999, 18, 1345–1356. [Google Scholar] [CrossRef] [Green Version]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001, 480–481, 243–268. [Google Scholar] [CrossRef]
- Chow, F.; Ozols, E.; Nikolic-Paterson, D.J.; Atkins, R.C.; Tesch, G.H. Macrophages in mouse type 2 diabetic nephropathy: Correlation with diabetic state and progressive renal injury. Kidney Int. 2004, 65, 116–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosugi, T.; Nakayama, T.; Heinig, M.; Zhang, L.; Yuzawa, Y.; Sanchez-Lozada, L.G.; Roncal, C.; Johnson, R.J.; Nakagawa, T. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am. J. Physiol. Ren. Physiol. 2009, 297, F481–F488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobé, G.; Willgoss, D.; Hogg, N.; Schoch, E.; Endre, Z. Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int. 1999, 56, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schena, F.P.; Grandaliano, G.; Gesualdo, L. The role of tubular cells in the progression of renal damage: Guilty or innocent? Ren. Fail. 2001, 23, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Pharmaceutical Name | Scientific Name | HJG | BJG |
|---|---|---|---|
| Radix Rehmanniae Preparata | Rehmannia glutinosa LIB. var. purpurea MAKINO | ✓ | ✓ |
| Rhizoma Dioscoreae | Dioscorea japonica THUNB. | ✓ | ✓ |
| Fructus Corni | Cornus officinalis SIEB. et ZUCC. | ✓ | ✓ |
| Hoelen | Poria cocos WOLF | ✓ | ✓ |
| Rhizoma Alismatis | Alisma plantago-aquatica L. subsp. orientale SAMUELSSON | ✓ | ✓ |
| Cortex Cinnamomi | Cinnamomum cassia BLUME | ✓ | ✓ |
| Cortex Moutan Radicis | Paeonia suffruticosa ANDREWS | ✓ | - |
| Radix Aconiti Lateralis Preparata | Aconitum carmichaeli DEBX. | ✓ | - |
| Fructus Schisandrae | Schisandra chinensis BAILLON | - | ✓ |
| Raidix Ophiopogonis | Ophiopogon japonicus KER-GAWLER var. genuinus MAXIM. | - | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.H.; Tanaka, T.; Akimoto, Y.; Jeon, J.P.; Yokozawa, T. Therapeutic Potential of Two Derivative Prescriptions of Rokumijiogan, Hachimijiogan and Bakumijiogan against Renal Damage in Nephrectomized Rats. Medicines 2023, 10, 24. https://doi.org/10.3390/medicines10030024
Park CH, Tanaka T, Akimoto Y, Jeon JP, Yokozawa T. Therapeutic Potential of Two Derivative Prescriptions of Rokumijiogan, Hachimijiogan and Bakumijiogan against Renal Damage in Nephrectomized Rats. Medicines. 2023; 10(3):24. https://doi.org/10.3390/medicines10030024
Chicago/Turabian StylePark, Chan Hum, Takashi Tanaka, Yoshie Akimoto, Jin Pyeong Jeon, and Takako Yokozawa. 2023. "Therapeutic Potential of Two Derivative Prescriptions of Rokumijiogan, Hachimijiogan and Bakumijiogan against Renal Damage in Nephrectomized Rats" Medicines 10, no. 3: 24. https://doi.org/10.3390/medicines10030024