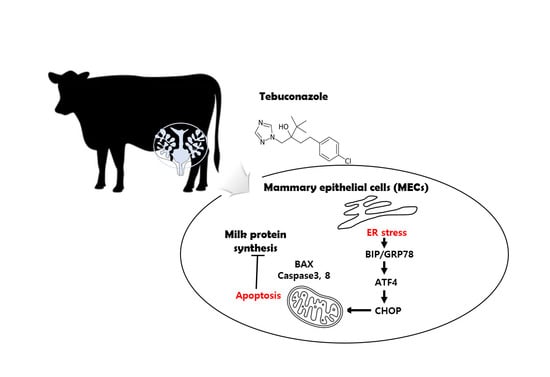
Tebuconazole Induces ER-Stress-Mediated Cell Death in Bovine Mammary Epithelial Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cytotoxicity Tests
2.3. Flow Cytometry
2.4. Immunostaining
2.5. RNA Isolation and Quantitative PCR
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. TEB Induced MAC-T Cell Apoptosis and Toxicity
3.2. Anti-Proliferation Effect of TEB on MAC-T Cell Culture
3.3. TEB Induces Mitochondrial Dysfunction in MAC-T Cells
3.4. TEB Induced Apoptosis via ER Stress in MAC-T Cells
3.5. TEB Regulated the Expression of Milk-Protein-Synthesis-Related Genes and Inflammatory Genes in MAC-T Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Ni, T.; Xie, F.; Hao, Y.; Yu, S.; Chai, X.; Jin, Y.; Wang, T.; Jiang, Y.; Zhang, D. Design, synthesis, and structure-activity relationship studies of novel triazole agents with strong antifungal activity against Aspergillus fumigatus. Bioorg. Med. Chem. Lett. 2020, 30, 126951. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Souders, C.L.; Li, P.; Pang, S.; Qiu, L.; Martyniuk, C.J. Developmental toxicity of the triazole fungicide cyproconazole in embryo-larval stages of zebrafish (Danio rerio). Environ. Sci. Pollut. Res. Int. 2019, 26, 4913–4923. [Google Scholar] [CrossRef] [PubMed]
- Svanholm, S.; Säfholm, M.; Brande-Lavridsen, N.; Larsson, E.; Berg, C. Developmental reproductive toxicity and endocrine activity of propiconazole in the Xenopus tropicalis model. Sci. Total Environ. 2021, 753, 141940. [Google Scholar] [CrossRef]
- Strickland, T.C.; Potter, T.L.; Joo, H. Tebuconazole dissipation and metabolism in Tifton loamy sand during laboratory incubation. Pest. Manag. Sci. 2004, 60, 703–709. [Google Scholar] [CrossRef]
- Lamb, D.C.; Kelly, D.E.; Manning, N.J.; Hollomon, D.W.; Kelly, S.L. Expression, purification, reconstitution and inhibition of Ustilago maydis sterol 14 alpha-demethylase (CYP51; P450(14DM). FEMS Microbiol. Lett. 1998, 169, 369–373. [Google Scholar]
- Sannino, A.; Bolzoni, L.; Bandini, M. Application of liquid chromatography with electrospray tandem mass spectrometry to the determination of a new generation of pesticides in processed fruits and vegetables. J. Chromatogr. A 2004, 1036, 161–169. [Google Scholar] [CrossRef]
- Li, Y.; Dong, F.; Liu, X.; Xu, J.; Li, J.; Kong, J.; Chen, X.; Liang, X.; Zheng, Y. Simultaneous enantioselective determination of triazole fungicides in soil and water by chiral liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2012, 1224, 51–60. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Zubrod, R.S.J.P.; Bundschuh, M.; Schulz, R. Effects of subchronic fungicide exposure on the energy processing of Gammarus fossarum (Crustacea; Amphipoda). Ecotoxicol. Environ. Saf. 2010, 73, 1674–1680. [Google Scholar] [CrossRef]
- Barone, S.; Moser, V.C. The effects of perinatal tebuconazole exposure on adult neurological, immunological, and reproductive function in rats. Toxicol. Sci. 2004, 77, 183. [Google Scholar] [CrossRef]
- Yang, J.D.; Liu, S.H.; Liao, M.H.; Chen, R.M.; Liu, P.Y.; Ueng, T.H. Effects of tebuconazole on cytochrome P450 enzymes, oxidative stress, and endocrine disruption in male rats. Environ. Toxicol. 2018, 19. [Google Scholar] [CrossRef]
- Pan, J.S.; Hong, M.J.; Ren, J.L. Reactive oxygen species: A double-edged sword in oncogenesis. World J. Gastroenterol. 2009, 15, 1702–1707. [Google Scholar] [CrossRef]
- Othmène, Y.B.; Monceaux, K.; Karoui, A.; Salem, I.B.; Belhadef, A.; Abid-Essefi, S.; Lemaire, C. Tebuconazole induces ROS-dependent cardiac cell toxicity by activating DNA damage and mitochondrial apoptotic pathway. Ecotoxicol. Environ. Saf. 2020, 204, 111040. [Google Scholar] [CrossRef]
- Ying, Y.; Pan, P.; Zou, C.; Wang, Y.; Tang, Y.; Hou, X.; Li, Y.; Xu, Q.; Lin, L.; Lu, J.; et al. Tebuconazole exposure disrupts placental function and causes fetal low birth weight in rats. Chemosphere 2021, 264, 128432. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Q.; Li, X.; Huang, T.; Wang, S.; Wang, Y.; Chen, X.; Lin, Z.; Ge, R.S. Pubertal exposure to tebuconazole increases testosterone production via inhibiting testicular aromatase activity in rats. Chemosphere 2019, 230, 519–526. [Google Scholar] [CrossRef]
- Capuco, A.V.; Wood, D.L.; Baldwin, R.; Mcleod, K.; Paape, M.J. Mammary cell number, proliferation, and apoptosis during a bovine lactation: Relation to milk production and effect of bST. J. Dairy. Sci. 2001, 84, 2177–2187. [Google Scholar] [CrossRef]
- Bruinenberg, M.; van Agtmaal, M.; Hoekstra, N.; van Eekeren, N. Residues of pesticides in dairy cow rations and fly treatments reduce the number of Coleoptera in dung. Agric. Ecosyst. Environ. 2023, 344, 108307. [Google Scholar] [CrossRef]
- Lee, W.H.; An, G.R.; Park, H.H.; Lim, W.S.; Song, G.H. Diflubenzuron leads to apoptotic cell death through ROS generation and mitochondrial dysfunction in bovine mammary epithelial cells. Pestic. Biochem. Physiol. 2021, 177, 104893. [Google Scholar] [CrossRef]
- Lee, H.J.; An, G.R.; Lim, W.H.; Song, G.H. Pendimethalin exposure induces bovine mammary epithelial cell death through excessive ROS production and alterations in the PI3K and MAPK signaling pathways. Pestic. Biochem. Physiol. 2022, 188, 105254. [Google Scholar] [CrossRef]
- Park, J.H.; An, G.R.; Lim, W.S.; Song, G.H. Aclonifen induces bovine mammary gland epithelial cell death by disrupting calcium homeostasis and inducing ROS production. Pestic. Biochem. Physiol. 2022, 181, 105011. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, R.; Yoo, H.J.; Hong, K.H.; Song, H. Nonylphenol Induces Apoptosis through ROS/JNK Signaling in a Spermatogonia Cell Line. Int. J. Mol. Sci. 2020, 30, 10307. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Heo, Y.T.; Lee, S.E.; Hwang, K.C.; Lee, H.G.; Choi, S.H.; Kim, N.H. Short communication: Retinoic acid plus prolactin to synergistically increase specific casein gene expression in MAC-T cells. J. Dairy Sci. 2013, 96, 3835–3839. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.; Buerge, I.J.; Hauser, A.; Müller, M.D.; Poiger, T. Azole fungicides: Occurrence and fate in wastewater and surface waters. Environ. Sci. Technol. 2008, 42, 7193–7200. [Google Scholar] [CrossRef]
- Haegler, P.; Joerin, L.; Krähenbühl, S.; Bouitbir, J. Hepatocellular Toxicity of Imidazole and Triazole Antimycotic Agents. Toxicol. Sci. 2017, 157, 183–195. [Google Scholar] [CrossRef]
- Othmène, Y.B.; Monceaux, K.; Belhadef, A.; Karoui, A.; Salem, I.B.; Boussabbeh, M.; Abid-Essefi, S.; Lemaire, C. Triazole fungicide tebuconazole induces apoptosis through ROS-mediated endoplasmic reticulum stress pathway. Environ. Toxicol. Pharmacol. 2022, 94, 103919. [Google Scholar] [CrossRef]
- Goetz, A.K.; Ren, H.; Schmid, J.E.; Blystone, C.R.; Thillainadarajah, I.; Best, D.S.; Nichols, H.P.; Strader, L.F.; Wolf, D.C.; Narotsky, M.G.; et al. Disruption of testosterone homeostasis as a mode of action for the reproductive toxicity of triazole fungicides in the male rat. Toxicol. Sci. 2007, 95, 227–239. [Google Scholar] [CrossRef]
- Chaâbane, M.; Tir, M.; Hamdi, S.; Boudawara, O.; Jamoussi, K.; Boudawara, T.; Ghorbel, R.E.; Zeghal, N.; Soudani, N. Improvement of Heart Redox States Contributes to the Beneficial Effects of Selenium Against Penconazole-Induced Cardiotoxicity in Adult Rats. Biol. Trace Elem. Res. 2016, 169, 261–270. [Google Scholar] [CrossRef]
- Saikumar, P.; Dong, Z.; Mikhailov, V.; Denton, M.; Weinberg, J.M.; Venkatachalam, M.A. Apoptosis: Definition, mechanisms, and relevance to disease. Am. J. Med. 1999, 107, 489–506. [Google Scholar] [CrossRef]
- Jan, R.; Saba, G.E.S. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling–from basic mechanisms to clinical applications. FEBS. J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Adams, J.M. Ways of dying: Multiple pathways to apoptosis. Genes Dev. 2003, 17, 2481–2495. [Google Scholar] [CrossRef]
- Othmène, Y.B.; Salem, I.B.; Hamdi, H.; Annabi, E.; Abid-Essefi, S. Tebuconazole induced cytotoxic and genotoxic effects in HCT116 cells through ROS generation. Pestic. Biochem. Physiol. 2021, 174, 104797. [Google Scholar] [CrossRef]
- Kwon, H.C.; Kim, D.H.; Jeong, C.H.; Kim, Y.J.; Han, J.H.; Lim, S.J.; Shin, D.M.; Kim, D.W.; Han, S.G. Tebuconazole Fungicide Induces Lipid Accumulation and Oxidative Stress in HepG2 Cells. Foods 2021, 22, 2242. [Google Scholar] [CrossRef]
- Othmène, Y.B.; Hamdi, J.; Salem, I.B.; Annabi, E.; Amara, I.; Neffati, F.; Najjar, M.F.; Abid-Essefi, S. Oxidative stress, DNA damage and apoptosis induced by tebuconazole in the kidney of male Wistar rat. Chem. Biol. Interact. 2020, 330, 109114. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Sun, Q.; Coffin, S.; Chen, L.; Qiao, K.; Gui, W.; Zhu, G. Tebuconazole induced oxidative stress related hepatotoxicity in adult and larval zebrafish (Danio rerio). Chemosphere 2020, 241, 125129. [Google Scholar] [CrossRef]
- Rzymski, T.; Milani, M.; Singleton, D.C.; Harris, A.L. Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia. Cell Cycle 2009, 8, 3838–3847. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 4, 3083. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death. Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 18, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jin, Y.; Zhao, Y.; Shan, A.; Fang, H.; Shen, J.; Zhou, C.; Yu, H.; Zhou, Y.; Wang, X.; et al. Zearalenone induces apoptosis in bovine mammary epithelial cells by activating endoplasmic reticulum stress. J. Dairy Sci. 2019, 102, 10543–10553. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Rennison, M.D.; Handel, S.E.; Wilde, C.J.; Burgoyne, R.D. Proteins are secreted by both constitutive and regulated secretory pathways in lactating mouse mammary epithelial cells. J. Cell Biol. 1992, 117, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.T.; Ha, W.T.; Lee, R.; Lee, W.Y.; Jeong, H.Y.; Hwang, K.C.; Song, H. Mammary alveolar cell as in vitro evaluation system for casein gene expression involved in glucose level. Asian-Australas. J. Anim. Sci. 2017, 30, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, A.; Verscheijden, L.F.M.; Mol, J.G.J.; Vermeulen, R.C.H.; Westerhout, J.; Roeleveld, N.; Russel, F.G.M.; Scheepers, P.T.J. Toxicokinetics of a urinary metabolite of tebuconazole following controlled oral and dermal administration in human volunteers. Arch. Toxicol. 2019, 93, 2545–2553. [Google Scholar] [CrossRef]
- Sanchez, C.L.; Sims, S.G.; Nowery, J.D.; Meares, G.P. Endoplasmic reticulum stress differentially modulates the IL-6 family of cytokines in murine astrocytes and macrophages. Sci. Rep. 2019, 17, 14931. [Google Scholar] [CrossRef]
- O’Reilly, S.; Ciechomska, M.; Cant, R.; van Laar, J.M. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J. Biol. Chem. 2014, 289, 9952–9960. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhao, L.; Pan, X.; Chen, N.N.; Chen, J.; Gong, Q.L.; Su, F.; Yan, J.; Zhang, Y.; Zhang, S.H. Hypoxia-stimulated cardiac fibroblast production of IL-6 promotes myocardial fibrosis via the TGF-β1 signaling pathway. Lab. Investig. 2016, 96, 839–852. [Google Scholar] [CrossRef]
- Elias, J.A.; Lentz, V.; Cummings, P.J. Transforming growth factor-beta regulation of IL-6 production by unstimulated and IL-1-stimulated human fibroblasts. J. Immunol. 1991, 146, 3437–3443. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Sukkar, M.B.; Khorasani, N.M.; Bhavsar, P.K.; Chung, K.F. TGF-β regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, 295–304. [Google Scholar] [CrossRef]
- Sheshadri, N.; Poria, D.K.; Sharan, S.; Hu, Y.; Yan, C.; Koparde, V.N.; Balamurugan, K.; Sterneck, E. PERK signaling through C/EBPδ contributes to ER stress-induced expression of immunomodulatory and tumor promoting chemokines by cancer cells. Cell Death. Dis. 2021, 1, 1038. [Google Scholar] [CrossRef]





| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | 5′-GGGTCATCATCTCTGCACCT-3′ | 5′-GGTCATAAGTCCCTCCACGA-3′ |
| CHOP | 5′-GCAACGCATGAAGGAGAAAG-3′ | 5′-AACCATCCGGTCAATCAGAG-3′ |
| GRP78 | 5′-TGGCTGGAAAGTCACCAAG-3′ | 5′-GTCTGCTGCTTCCTCCTCAC-3′ |
| ATF4 | 5′-GCTGTGGATTGGTTGGTCTC-3′ | 5′-AGCTCATCTGGCAT-3′ |
| LGB | 5′-CTTGTGCTGGACACCGACTA-3′ | 5′-TTGAGGGCTTTGTCGAATTT-3′ |
| LALA | 5′-AAAGACGACCAGAACCCTCA-3′ | 5′-GCTTTATGGGCCAACCAGTA-3′ |
| CSN1S1 | 5′-CACTGAGTCAAAGGGAATTAAAG-3′ | 5′-TGATGGCACTTACAGGAGA-3′ |
| CSN1S2 | 5′-CCTAACAGCCTCCCACAAAA-3′ | 5′-AGACTGGAGCAGAGGCAGAG-3′ |
| CSNK | 5′-CCAGGAGCAAAACCAAGAAC-3′ | 5′-TGCAACTGGTTTCTGTTGGT-3′ |
| TGFB3 | 5′-TCTGGGGCGACTTAAGAAGA-3′ | 5′-ATTGCGGAAGCAGTAATTGG-3′ |
| CEBPD | 5′-ATCGACTTCAGCGCCTACAT-3′ | 5′-TGTGGTTGCTGTTGAAGAGG-3′ |
| IL6 | 5′-AAGCAGCAAGGAGACACTGG-3′ | 5′-GCCTGATTGAACCCAGATTG-3′ |
| Antibody | Manufacturer | Catalog Number | Dilution (Usage) |
|---|---|---|---|
| Cleaved-caspase3 | Cell signaling(Danvers, MA, USA) | #9664S | 1:2000 (WB) |
| Cleaved-capase8 | Cell signaling | #8592S | 1:2000 (WB) |
| BAX | Santa Cruz(Dallas, Texas, USA) | Sc-7480 | 1:2000 (WB) |
| BIP/GRP78 | Cell signaling | #3177 | 1:2000 (WB) |
| ERO-La | Cell signaling | #377009 | 1:2000 (WB) |
| PDI | Cell signaling | #3501 | 1:2000 (WB) |
| β-actin | Santa Cruz | Ab16667 | 1:2000 (WB) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-Y.; Lee, R.; Park, H.-J. Tebuconazole Induces ER-Stress-Mediated Cell Death in Bovine Mammary Epithelial Cell Lines. Toxics 2023, 11, 397. https://doi.org/10.3390/toxics11040397
Lee W-Y, Lee R, Park H-J. Tebuconazole Induces ER-Stress-Mediated Cell Death in Bovine Mammary Epithelial Cell Lines. Toxics. 2023; 11(4):397. https://doi.org/10.3390/toxics11040397
Chicago/Turabian StyleLee, Won-Young, Ran Lee, and Hyun-Jung Park. 2023. "Tebuconazole Induces ER-Stress-Mediated Cell Death in Bovine Mammary Epithelial Cell Lines" Toxics 11, no. 4: 397. https://doi.org/10.3390/toxics11040397