Microplastic Presence in the Digestive Tract of Pearly Razorfish Xyrichtys novacula Causes Oxidative Stress in Liver Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Microplastic Analysis
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Biometric Parameters
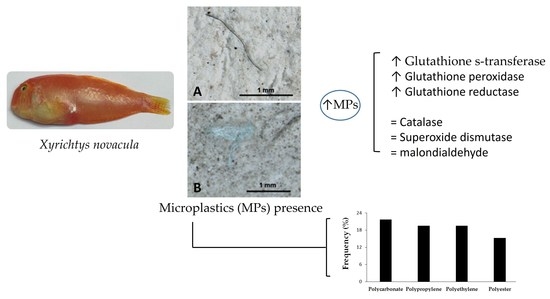
3.2. Microplastics in the Gastrointestinal Tract
3.3. Biomarker Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Schmaltz, E.; Melvin, E.C.; Diana, Z.; Gunady, E.F.; Rittschof, D.; Somarelli, J.A.; Virdin, J.; Dunphy-Daly, M.M. Plastic Pollution Solutions: Emerging Technologies to Prevent and Collect Marine Plastic Pollution. Environ. Int. 2020, 144, 106067. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Plastic Debris in the Marine Environment: History and Future Challenges. Glob. Chall. 2020, 4, 1900081. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Höök, T.O. A Meta-Analysis of the Effects of Exposure to Microplastics on Fish and Aquatic Invertebrates. Sci. Total Environ. 2018, 631–632, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Lefebvre, C.; Saraux, C.; Heitz, O.; Nowaczyk, A.; Bonnet, D. Microplastics FTIR Characterisation and Distribution in the Water Column and Digestive Tracts of Small Pelagic Fish in the Gulf of Lions. Mar. Pollut. Bull. 2019, 142, 510–519. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Shi, Y.; Zhao, Y.; Zheng, S.; Liang, J.; Liu, T.; Tian, Z. Characteristics and Retention of Microplastics in the Digestive Tracts of Fish from the Yellow Sea. Environ. Pollut. 2019, 249, 878–885. [Google Scholar] [CrossRef]
- Wu, J.; Lai, M.; Zhang, Y.; Li, J.; Zhou, H.; Jiang, R.; Zhang, C. Microplastics in the Digestive Tracts of Commercial Fish from the Marine Ranching in East China Sea, China. Case Stud. Chem. Environ. Eng. 2020, 2, 100066. [Google Scholar] [CrossRef]
- Fagiano, V.; Alomar, C.; Compa, M.; Soto-Navarro, J.; Jordá, G.; Deudero, S. Neustonic Microplastics and Zooplankton in Coastal Waters of Cabrera Marine Protected Area (Western Mediterranean Sea). Sci. Total Environ. 2022, 804, 150120. [Google Scholar] [CrossRef]
- Prokić, M.D.; Gavrilović, B.R.; Radovanović, T.B.; Gavrić, J.P.; Petrović, T.G.; Despotović, S.G.; Faggio, C. Studying Microplastics: Lessons from Evaluated Literature on Animal Model Organisms and Experimental Approaches. J. Hazard. Mater. 2021, 414, 125476. [Google Scholar] [CrossRef]
- Lavers, J.L.; Hutton, I.; Bond, A.L. Clinical Pathology of Plastic Ingestion in Marine Birds and Relationships with Blood Chemistry. Environ. Sci. Technol. 2019, 53, 9224–9231. [Google Scholar] [CrossRef] [PubMed]
- Gall, S.C.; Thompson, R.C. The Impact of Debris on Marine Life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.G. Entanglement of Birds in Plastics and Other Synthetic Materials. Mar. Pollut. Bull. 2018, 135, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, E.M.; de Bruyn, P.J.N. Pinniped Entanglement in Oceanic Plastic Pollution: A Global Review. Mar. Pollut. Bull. 2019, 145, 295–305. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and Effects of Plastic Additives on Marine Environments and Organisms: A Review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and Health Hazard Ranking and Assessment of Plastic Polymers Based on Chemical Composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Lewis, P.N.; Riddle, M.J.; Smith, S.D.A. Assisted Passage or Passive Drift: A Comparison of Alternative Transport Mechanisms for Non-Indigenous Coastal Species into the Southern Ocean. Antarct. Sci. 2005, 17, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef]
- Mancia, A.; Chenet, T.; Bono, G.; Geraci, M.L.; Vaccaro, C.; Munari, C.; Mistri, M.; Cavazzini, A.; Pasti, L. Adverse Effects of Plastic Ingestion on the Mediterranean Small-Spotted Catshark (Scyliorhinus canicula). Mar. Environ. Res. 2020, 155, 104876. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Álvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-Term Exposure to Microplastics Induces Oxidative Stress and a pro-Inflammatory Response in the Gut of Sparus Aurata Linnaeus, 1758. Environ. Pollut. 2020, 266, 115295. [Google Scholar] [CrossRef]
- Hodkovicova, N.; Hollerova, A.; Svobodova, Z.; Faldyna, M.; Faggio, C. Effects of Plastic Particles on Aquatic Invertebrates and Fish—A Review. Environ. Toxicol. Pharmacol. 2022, 96, 104013. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and Diseases: Role in Metabolism and Energy Supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant Enzymes and Human Diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic Ingestion by Mullus Surmuletus Linnaeus, 1758 Fish and Its Potential for Causing Oxidative Stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione Transferases: Substrates, Inihibitors and pro-Drugs in Cancer and Neurodegenerative Diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Sureda, A.; Box, A.; Enseñat, M.; Alou, E.; Tauler, P.; Deudero, S.; Pons, A. Enzymatic Antioxidant Response of a Labrid Fish (Coris julis) Liver to Environmental Caulerpenyne. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 144, 191–196. [Google Scholar] [CrossRef]
- Delaeter, C.; Spilmont, N.; Bouchet, V.M.P.; Seuront, L. Plastic Leachates: Bridging the Gap between a Conspicuous Pollution and Its Pernicious Effects on Marine Life. Sci. Total Environ. 2022, 826, 154091. [Google Scholar] [CrossRef]
- Solomando, A.; Cohen-Sánchez, A.; Box, A.; Montero, I.; Pinya, S.; Sureda, A. Microplastic Presence in the Pelagic Fish, Seriola dumerili, from Balearic Islands (Western Mediterranean), and Assessment of Oxidative Stress and Detoxification Biomarkers in Liver. Environ. Res. 2022, 212, 113369. [Google Scholar] [CrossRef]
- Hoyo-Alvarez, E.; Arechavala-Lopez, P.; Jiménez-García, M.; Solomando, A.; Alomar, C.; Sureda, A.; Moranta, D.; Deudero, S. Effects of Pollutants and Microplastics Ingestion on Oxidative Stress and Monoaminergic Activity of Seabream Brains. Aquat. Toxicol. 2022, 242, 106048. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of Polystyrene Microplastics in Juvenile Eriocheir Sinensis and Oxidative Stress Effects in the Liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl Chloride Microplastics Induce Growth Inhibition and Oxidative Stress in Cyprinus carpio Var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, Y.B.; Choi, J.H. Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Neurotoxicity in Fish Exposed to Microplastics: A Review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef]
- Hsieh, S.L.; Wu, Y.C.; Xu, R.Q.; Chen, Y.T.; Chen, C.W.; Singhania, R.R.; Dong, C. Di Effect of Polyethylene Microplastics on Oxidative Stress and Histopathology Damages in Litopenaeus vannamei. Environ. Pollut. 2021, 288, 117800. [Google Scholar] [CrossRef]
- Castriota, L.; Scarabello, M.P.; Finoia, M.G.; Sinopoli, M.; Andaloro, F. Food and Feeding Habits of Pearly Razorfish, Xyrichtys novacula (Linnaeus, 1758), in the Southern Tyrrhenian Sea: Variation by Sex and Size. Environ. Biol. Fishes 2005, 72, 123–133. [Google Scholar] [CrossRef]
- Del Valle, L.; Murray, I.; Pons, G.X.; Calvo, J. Capacidad de Carga Socioambiental de la Isla de Eivissa. Estado de La Cuestión; Societat d’Història Natural de les Balears: Mallorca, Spain, 2017; Volume 26, pp. 68–83. [Google Scholar]
- Beltrano, A.M.; Cannizzaro, L.; Vitale, S.; Milazzo, A. Preliminary study on the feeding habits of cleaver wrasse Xyrichtys novacula (Pisces: Labridae) in the Strait of Sicily (Mediterranean Sea). Electron. J. Ichthyol. 2006, 2, 50–54. [Google Scholar]
- Cardinale, M.; Colloca, F.; Ardizzone, G.D. Feeding Ecology of Mediterranean Razorfish Xyrichthys novacula in the Tyrrhenian Sea (Central Mediterranean Sea). J. Appl. Ichthyol. 1997, 13, 105–111. [Google Scholar] [CrossRef]
- Katsanevakis, S. Habitat Use by the Pearly Razorfish, Xyrichtys novacula (Pisces: Labridae). Sci. Mar. 2005, 69, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Alós, J.; Cabanellas-Reboredo, M.; Lowerre-Barbieri, S. Diel Behaviour and Habitat Utilisation by the Pearly Razorfish during the Spawning Season. Mar. Ecol. Prog. Ser. 2012, 460, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Castriota, L.; Falautano, M.; Finoia, M.G.; Campo, D.; Scarabello, M.P.; Andaloro, F. Temporal Variations in the Diet of Pearly Razorfish Xyrichtys novacula (Osteichthyes: Labridae). J. Fish Biol. 2010, 76, 1626–1639. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of Microplastics by Commercial Fish off the Portuguese Coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takada, H. Microplastic Fragments and Microbeads in Digestive Tracts of Planktivorous Fish from Urban Coastal Waters. Sci. Rep. 2016, 6, 34351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Li, Q.; Shi, Y.; Zheng, S.; Liang, J.; Liu, T.; Tian, Z. Data on Microplastics in the Digestive Tracts of 19 Fish Species from the Yellow Sea, China. Data Br. 2019, 25, 103989. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, H.; Chen, B.; Sun, X.; Qu, K.; Xia, B. Microplastic Ingestion in Deep-Sea Fish from the South China Sea. Sci. Total Environ. 2019, 677, 493–501. [Google Scholar] [CrossRef]
- Cohen-Sánchez, A.; Solomando, A.; Pinya, S.; Tejada, S.; Valencia, J.M.; Box, A.; Sureda, A. First Detection of Microplastics in Xyrichtys novacula (Linnaeus 1758) Digestive Tract from Eivissa Island (Western Mediterranean). Environ. Sci. Pollut. Res. 2022, 29, 65077–65087. [Google Scholar] [CrossRef]
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High Quantities of Microplastic in Arctic Deep-Sea Sediments from the HAUSGARTEN Observatory. Environ. Sci. Technol. 2017, 51, 11000–11010. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Flohé, L.; Ötting, F. Superoxide Dismutase Assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Cardone, F.; Alifano, P.; Tredici, S.M.; Piraino, S.; Corriero, G.; Gaino, E. Epidemic Mortality of the Sponge Ircinia variabilis (Schmidt, 1862) Associated to Proliferation of a Vibrio Bacterium. Microb. Ecol. 2012, 64, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Markic, A.; Gaertner, J.C.; Gaertner-Mazouni, N.; Koelmans, A.A. Plastic Ingestion by Marine Fish in the Wild. Crit. Rev. Environ. Sci. Technol. 2020, 50, 657–697. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, Tissue Distribution, and Biochemical Effects of Polystyrene Microplastics in the Freshwater Fish Red Tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Jovanović, B.; Gökdağ, K.; Güven, O.; Emre, Y.; Whitley, E.M.; Kideys, A.E. Virgin Microplastics Are Not Causing Imminent Harm to Fish after Dietary Exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.; Raimundo, J.; Caetano, M.; Garrido, S. Microplastic Ingestion and Diet Composition of Planktivorous Fish. Limnol. Oceanogr. Lett. 2020, 5, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Nadal, M.A.; Alomar, C.; Deudero, S. High Levels of Microplastic Ingestion by the Semipelagic Fish Bogue Boops boops (L.) around the Balearic Islands. Environ. Pollut. 2016, 214, 517–523. [Google Scholar] [CrossRef]
- Pellini, G.; Gomiero, A.; Fortibuoni, T.; Ferrà, C.; Grati, F.; Tassetti, A.N.; Polidori, P.; Fabi, G.; Scarcella, G. Characterization of Microplastic Litter in the Gastrointestinal Tract of Solea solea from the Adriatic Sea. Environ. Pollut. 2018, 234, 943–952. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic Litter Composition of the Turkish Territorial Waters of the Mediterranean Sea, and Its Occurrence in the Gastrointestinal Tract of Fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Box, A.; Grau, A.M.; Blanco, A.; Riera, F. Els Raors (Xyrichthys novacula) a La Reserva Dels Freus d’Eivissa i Formentera; Efecte de La Protecció Espacial. Boll. Soc. Historia Nat. Balear. 2009, 52, 193–201. [Google Scholar]
- López-Martínez, S.; Morales-Caselles, C.; Kadar, J.; Rivas, M.L. Overview of Global Status of Plastic Presence in Marine Vertebrates. Glob. Chang. Biol. 2021, 27, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe Scad Decapterus muroadsi (Carangidae) Fish Ingest Blue Microplastics Resembling Their Copepod Prey along the Coast of Rapa Nui (Easter Island) in the South Pacific Subtropical Gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Fagiano, V.; Compa, M.; Alomar, C.; Rios-Fuster, B.; Morató, M.; Capó, X.; Deudero, S. Breaking the Paradigm: Marine Sediments Hold Two-Fold Microplastics than Sea Surface Waters and Are Dominated by Fibers. Sci. Total Environ. 2023, 858, 159722. [Google Scholar] [CrossRef]
- Hartline, N.L.; Bruce, N.J.; Karba, S.N.; Ruff, E.O.; Sonar, S.U.; Holden, P.A. Microfiber Masses Recovered from Conventional Machine Washing of New or Aged Garments. Environ. Sci. Technol. 2016, 50, 11532–11538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koongolla, J.B.; Lin, L.; Pan, Y.F.; Yang, C.P.; Sun, D.R.; Liu, S.; Xu, X.R.; Maharana, D.; Huang, J.S.; Li, H.X. Occurrence of Microplastics in Gastrointestinal Tracts and Gills of Fish from Beibu Gulf, South China Sea. Environ. Pollut. 2020, 258, 113734. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Aceves, M.A.; Abo-Al-Ela, H.G.; Faggio, C. Physiological and Metabolic Approach of Plastic Additive Effects: Immune Cells Responses. J. Hazard. Mater. 2021, 404, 124114. [Google Scholar] [CrossRef]
- Gomes, M.T.R.; Guimarães, E.S.; Marinho, F.V.; Macedo, I.; Aguiar, E.R.G.R.; Barber, G.N.; Moraes-Vieira, P.M.M.; AlvesFilho, J.C.; Oliveira, S.C. STING Regulates Metabolic Reprogramming in Macrophages via HIF-1α during Brucella Infection. PLoS Pathog. 2021, 17, 1–25. [Google Scholar] [CrossRef]
- Lushchak, V.I. Contaminant-Induced Oxidative Stress in Fish: A Mechanistic Approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar] [CrossRef]
- Ribeiro, F.; O’Brien, J.W.; Galloway, T.; Thomas, K. V Accumulation and Fate of Nano- and Micro-Plastics and Associated Contaminants in Organisms. TrAC Trends Anal. Chem. 2019, 111, 139–147. [Google Scholar] [CrossRef]
- Li, L.; Gu, H.; Chang, X.; Huang, W.; Sokolova, I.M.; Wei, S.; Sun, L.; Li, S.; Wang, X.; Hu, M.; et al. Oxidative Stress Induced by Nanoplastics in the Liver of Juvenile Large Yellow Croaker Larimichthys crocea. Mar. Pollut. Bull. 2021, 170, 112661. [Google Scholar] [CrossRef] [PubMed]
- Agus, H.H.; Sümer, S.; Erkoç, F. Toxicity and Molecular Effects of Di-n-Butyl Phthalate (DBP) on CYP1A, SOD, and GPx in Cyprinus carpio (Common Carp). Environ. Monit. Assess. 2015, 187, 423. [Google Scholar] [CrossRef]
- Zheng, Q.; Feng, M.; Dai, Y. Comparative Antioxidant Responses in Liver of Carassius Auratus Exposed to Phthalates: An Integrated Biomarker Approach. Environ. Toxicol. Pharmacol. 2013, 36, 741–749. [Google Scholar] [CrossRef]
- Lombardo, J.; Solomando, A.; Cohen-Sánchez, A.; Pinya, S.; Tejada, S.; Ferriol, P.; Mateu-Vicens, G.; Box, A.; Faggio, C.; Sureda, A. Effects of Human Activity on Markers of Oxidative Stress in the Intestine of Holothuria Tubulosa, with Special Reference to the Presence of Microplastics. Int. J. Mol. Sci. 2022, 23, 9018. [Google Scholar] [CrossRef]
- Capó, X.; Company, J.J.; Alomar, C.; Compa, M.; Sureda, A.; Grau, A.; Hansjosten, B.; López-Vázquez, J.; Quintana, J.B.; Rodil, R.; et al. Long-Term Exposure to Virgin and Seawater Exposed Microplastic Enriched-Diet Causes Liver Oxidative Stress and Inflammation in Gilthead Seabream Sparus aurata, Linnaeus 1758. Sci. Total Environ. 2021, 767, 144976. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, N.; Cappello, T.; Missawi, O.; Boughattas, I.; De Marco, G.; Belbekhouche, S.; Mokni, M.; Alphonse, V.; Guerbej, H.; Bousserrhine, N.; et al. Metabolomic Disorders Unveil Hepatotoxicity of Environmental Microplastics in Wild Fish Serranus scriba (Linnaeus 1758). Sci. Total Environ. 2022, 838, 155872. [Google Scholar] [CrossRef] [PubMed]
- Capó, X.; Morató, M.; Alomar, C.; Rios-Fuster, B.; Valls, M.; Compa, M.; Deudero, S. A Biomarker Approach as Responses of Bioindicator Commercial Fish Species to Microplastic Ingestion: Assessing Tissue and Biochemical Relationships. Biology 2022, 11, 1634. [Google Scholar] [CrossRef] [PubMed]
- Compa, M.; Alomar, C.; Morató, M.; Álvarez, E.; Deudero, S. Spatial Distribution of Macro- and Micro-Litter Items along Rocky and Sandy Beaches of a Marine Protected Area in the Western Mediterranean Sea. Mar. Pollut. Bull. 2022, 178, 113520. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen-Sánchez, A.; Solomando, A.; Pinya, S.; Tejada, S.; Valencia, J.M.; Box, A.; Sureda, A. Microplastic Presence in the Digestive Tract of Pearly Razorfish Xyrichtys novacula Causes Oxidative Stress in Liver Tissue. Toxics 2023, 11, 365. https://doi.org/10.3390/toxics11040365
Cohen-Sánchez A, Solomando A, Pinya S, Tejada S, Valencia JM, Box A, Sureda A. Microplastic Presence in the Digestive Tract of Pearly Razorfish Xyrichtys novacula Causes Oxidative Stress in Liver Tissue. Toxics. 2023; 11(4):365. https://doi.org/10.3390/toxics11040365
Chicago/Turabian StyleCohen-Sánchez, Amanda, Antònia Solomando, Samuel Pinya, Silvia Tejada, José María Valencia, Antonio Box, and Antoni Sureda. 2023. "Microplastic Presence in the Digestive Tract of Pearly Razorfish Xyrichtys novacula Causes Oxidative Stress in Liver Tissue" Toxics 11, no. 4: 365. https://doi.org/10.3390/toxics11040365