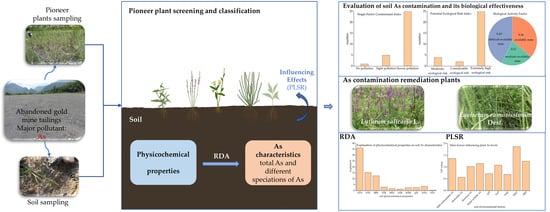
Characteristics of Soil Arsenic Contamination and the Potential of Pioneer Plants for Arsenic Remediation in Gold Mine Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil and Plant Sample Collection
2.3. Soil and Plant Data Determination
2.4. Evaluation of Soil As Contamination and Its Biological Effectiveness
2.4.1. Single-Factor Contaminant Index (Pi)
2.4.2. Potential Ecological Risk Index (PERI)
2.4.3. Biological Activity Factor
2.5. Enrichment and Transport Ability of Plants for As
2.5.1. Bioaccumulation Factor (BCF)
2.5.2. Translocation Factor (TF)
2.6. Analysis of Influencing Factors
2.6.1. Pearson Correlation Analysis and Redundancy Analysis (RDA)
2.6.2. Partial Least Squares Regression Model (PLSR)
2.7. Data Statistics and Analysis
3. Results
3.1. Soil Physicochemical Characteristics
3.2. Characterization of Soil As Contamination
3.2.1. Speciation and Concentration of As in Soil
3.2.2. Soil As Contamination Evaluation and Bioavailability Analysis
3.2.3. Correlation between Soil As and Physicochemical Properties
3.3. As Characterization of Pioneer Plants
3.3.1. As Concentration in Pioneer Plants
3.3.2. Analysis of Pioneer Plants’ Ability to Enrich and Transport As
3.3.3. Correlation of As Levels in Pioneer Plants with Soil Environmental Factors
4. Discussion
4.1. Effects of Physicochemical Properties on As Characterization in Soil
4.2. Classification of the Accumulation Characteristics of Pioneer Plants for As
4.3. Factors Influencing As Enrichment in Plants
4.4. Measures for Remediation of As-Contaminated Tailing
4.5. Deficiency and Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamaki, S.; Frankenberger, W.T., Jr. Environmental biochemistry of arsenic. Rev. Environ. Contam. Toxicol. 1992, 124, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Shabanov, M.V.; Marichev, M.S.; Minkina, T.M.; Mandzhieva, S.S.; Nevidomskaya, D.G. Assessment of the Impact of Industry-Related Air Emission of Arsenic in the Soils of Forest Ecosystems. Forests 2023, 14, 632. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; De Silva, P.M.C. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Khan, R.; Khan, A.; Qamar, W.; Arafah, A.; Ahmad, A.; Ahmad, A.; Akhter, R.; Rinklebe, J.; Ahmad, P. Fate of arsenic in living systems: Implications for sustainable and safe food chains. J. Hazard. Mater. 2021, 417, 126050. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Wai, K.M.; Wu, S.; Li, X.; Jaffe, D.A.; Perry, K.D. Global atmospheric transport and source-receptor relationships for arsenic. Environ. Sci. Technol. 2016, 50, 3714–3720. [Google Scholar] [CrossRef] [PubMed]
- Han, F.X.; Su, Y.; Monts, D.L.; Plodinec, M.J.; Banin, A.; Triplett, G.E. Assessment of global industrial-age anthropogenic arsenic contamination. Naturwissenschaften 2003, 90, 395–401. [Google Scholar] [CrossRef]
- Tang, J.W.; Liao, Y.P.; Yang, Z.H.; Chai, L.Y.; Yang, W.C. Characterization of arsenic serious-contaminated soils from Shimen realgar mine area, the Asian largest realgar deposit in China. J. Soils Sediments 2016, 16, 1519–1528. [Google Scholar] [CrossRef]
- Liu, G.; Shi, Y.; Guo, G.L.; Zhao, L.; Niu, J.J.; Zhang, C. Soil pollution characteristics and systemic environmental risk assessment of a large-scale arsenic slag contaminated site. J. Cleaner Prod. 2020, 251, 119721. [Google Scholar] [CrossRef]
- Xing, Y.; Brugger, J.; Tomkins, A.; Shvarov, Y. Arsenic evolution as a tool for understanding formation of pyritic gold ores. Geology 2019, 47, 335–338. [Google Scholar] [CrossRef]
- Kusin, F.M.; Awang, N.H.C.; Hasan, S.N.M.S.; Rahim, H.A.A.; Azmin, N.; Jusop, S.; Kim, K.W. Geo-ecological evaluation of mineral, major and trace elemental composition in waste rocks, soils and sediments of a gold mining area and potential associated risks. Catena 2019, 183, 104229. [Google Scholar] [CrossRef]
- Wen, Q.Q.; Yang, X.; Yan, X.L.; Yang, L.S. Evaluation of arsenic mineralogy and geochemistry in gold mine-impacted matrices: Speciation, transformation, and potential associated risks. J. Environ. Manag. 2022, 308, 114619. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Casey, R.J.; Caridi, D. The management of arsenic wastes: Problems and prospects. J. Hazard. Mater. 2000, 76, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Meunier, L.; Walker, S.R.; Wragg, J.; Parsons, M.B.; Koch, I.; Jamieson, H.E.; Reimer, K.J. Effects of soil composition and mineralogy on the bioaccessibility of arsenic from tailings and soil in gold mine districts of Nova Scotia. Environ. Sci. Technol. 2010, 44, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Qin, H. Bioconcentration and translocation of heavy metals in the soil-plants system in Machangqing copper mine, Yunnan Province, China. J. Geochem. Explor. 2019, 200, 159–166. [Google Scholar] [CrossRef]
- Costa, M.R.; Gošar, D.; Pinti, M.; Ferreira, A.; Marušič, M.B. In vitro toxicity of arsenic rich waters from an abandoned gold mine in northeast Portugal. Environ. Res. 2021, 202, 111683. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ji, B.; Khoso, S.A.; Tang, H.H.; Liu, R.Q.; Wang, L.; Hu, Y.H. An extensive review on restoration technologies for mining tailings. Environ. Sci. Pollut. Res. 2018, 25, 33911–33925. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.H.; Liu, R.Q.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef]
- Dubchak, S.; Bondar, O. Bioremediation and phytoremediation: Best approach for rehabilitation of soils for future use. In Remediation Measures for Radioactively Contaminated Areas; Springer: Cham, Switzerland, 2019; pp. 201–221. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2019, 249, 71–131. [Google Scholar] [CrossRef]
- Álvarez-Mateos, P.; Alés-ÁlvarezJuan, F.J.; García-Martín, F. Phytoremediation of highly contaminated mining soils by Jatropha curcas L. and production of catalytic carbons from the generated biomass. J. Environ. Manag. 2019, 231, 886–895. [Google Scholar] [CrossRef]
- Meharg, A.A. Variation in arsenic accumulation–hyperaccumulation in ferns and their allies: Rapid report. New Phytol. 2003, 157, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Arsenic hyperaccumulation by different fern species. New Phytol. 2002, 156, 27–31. [Google Scholar] [CrossRef]
- Zheng, M.X.; Xu, J.M.; Smith, L.; Naidu, R. Why a fern (Pteris multifida) dominantly growing on an arsenictheavy metal contamînated soil does not accumulate arsenic? J. Phys. IV 2003, 107, 1409–1411. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Ewel, J.J.; Putz, F.E. A place for alien species in ecosystem restoration. Front. Ecol. Environ. 2004, 2, 354–360. [Google Scholar] [CrossRef]
- Mensah, A.K.; Shaheen, S.M.; Rinklebe, J.; Heinze, S.; Marschner, B. Phytoavailability and uptake of arsenic in ryegrass affected by various amendments in soil of an abandoned gold mining site. Environ. Res. 2022, 214, 113729. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Wu, T.; Bao, H.X.; Liu, X.Y.; Xu, C.L.; Zhao, Y.N.; Liu, D.Y.; Yu, H.T. Potential toxic trace element (PTE) contamination in Baoji urban soil (NW China): Spatial distribution, mobility behavior, and health risk. Environ. Sci. Pollut. Res. 2017, 4, 24–19749. [Google Scholar] [CrossRef]
- Shrivastava, A.; Ghosh, D.; Dash, A.; Bose, S. Arsenic Contamination in Soil and Sediment in India: Sources, Effects, and Remediation. Curr. Pollut. Rep. 2015, 1, 35–46. [Google Scholar] [CrossRef]
- Kim, E.J.; Yoo, J.C.; Baek, K. Arsenic speciation and bioaccessibility in arsenic-contaminated soils: Sequential extraction and mineralogical investigation. Environ. Pollut. 2014, 186, 29–35. [Google Scholar] [CrossRef]
- Bradham, K.D.; Scheckel, K.G.; Nelson, C.M.; Seales, P.E.; Lee, G.E.; Hughes, M.F.; Miller, B.W.; Yeow, A.; Gilmore, T.; Serda, S.M.; et al. Relative bioavailability and bioaccessibility and speciation of arsenic in contaminated soils. Environ. Health Perspect. 2011, 119, 1629–1634. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, X.B.; Zhang, H.; Li, Y.T.; Zhao, S.Z.; Su, S.M.; Bai, L.Y.; Wang, Y.N.; Zhang, T. Effect of exogenous phosphate on the lability and phytoavailability of arsenic in soils. Chemosphere 2018, 196, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Karimi, N.; Sandalio, L.M. Arsenic hyperaccumulation strategies: An overview. Front. Cell Dev. Biol. 2017, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, R.A.; Myers, N.; Thomsen, J.B.; Da Fonseca, G.A.; Olivieri, S. Biodiversity hotspots and major tropical wilderness areas: Approaches to setting conservation priorities. Conserv. Biol. 1998, 12, 516–520. [Google Scholar] [CrossRef]
- Zhang, H.J.; Gao, Y.; Hua, Y.W.; Zhang, Y.; Liu, K. Assessing and mapping recreationists’ perceived social values for ecosystem services in the Qinling Mountains, China. Ecosyst. Serv. 2019, 39, 101006. [Google Scholar] [CrossRef]
- Wang, B.; Xu, G.C.; Li, P.; Li, Z.B.; Zhang, Y.X.; Cheng, Y.T.; Jia, L.; Zhang, J.X. Vegetation dynamics and their relationships with climatic factors in the Qinling Mountains of China. Ecol. Indic. 2020, 108, 105719. [Google Scholar] [CrossRef]
- Liu, S.D.; Xue, B.R.; Gao, J. The ecological security research of the northern slope of Qinling mountains based on the DPSIR model. Adv. Mater. Res. 2014, 1073, 438–444. [Google Scholar] [CrossRef]
- Li, P. Meeting the environmental challenges. Hum. Ecol. Risk Assess. 2020, 26, 2303–2315. [Google Scholar] [CrossRef]
- HJ 962-2018; Soil-Determination of pH-Potentiometry. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2019. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201808/t20180815_451430.shtml (accessed on 1 January 2019).
- NY/T 1121.4-2006; Soil Testing: Part 4: Method for Determination of Soil Bulk Density. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2006. Available online: http://down.foodmate.net/standard/yulan.php?itemid=10718 (accessed on 1 October 2006).
- HJ 613-2011; Soil-Determination of Dry Matter and Water Content-Gravimetric Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2011. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201104/t20110422_209587.shtml (accessed on 1 October 2011).
- Chen, R.; Han, L.; Zhao, Y.H.; Liu, Z.; Fan, Y.M.; Li, R.S.; Xia, L.F. Response of plant element traits to soil arsenic stress and its implications for vegetation restoration in a post-mining area. Ecol. Indic. 2023, 146, 109931. [Google Scholar] [CrossRef]
- DB 61/T 902.5-2013; Determination of Heavy Metal in Plant Extracts-Determination of Arsenic. Shaanxi Provincial Bureau of Quality and Technical Supervision: Xi’an, China, 2013. Available online: http://down.foodmate.net/standard/yulan.php?itemid=62618 (accessed on 1 March 2014).
- HJ 680-2013; Soil and Sediment-Determination of Mercury, Arsenic, Selenium, Bismuth, Antimony-Microwave Dissolution/Atomic Fluorescence Spectrometry. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2014. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201312/t20131203_264304.shtml (accessed on 1 February 2014).
- GB/T 25282-2010; Soil and Sediment—Sequential Extraction Procedure of Speciation of 13 Trace Elements. China National Standardization Management Committee, General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2011. Available online: http://c.gb688.cn/bzgk/gb/showGb?type=online&hcno=3CD527C889212919CFEC80932D514455 (accessed on 1 February 2011).
- Gololobova, A.; Legostaeva, Y. An Assessment of the Impact of the Mining Industry on Soil and Plant Contamination by Potentially Toxic Elements in Boreal Forests. Forests 2023, 14, 1641. [Google Scholar] [CrossRef]
- GB 36600-2018; Soil Environmental Quality–Risk Control Standard for Soil Contamination of a Development Land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/trhj/201807/t20180703_446027.shtml (accessed on 1 August 2018).
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Xu, Q.S.; Wang, J.M.; Shi, W.T. Source apportionment and potential ecological risk assessment of heavy metals in soils on a large scale in China. Environ. Geochem. Health 2023, 45, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Sabet Aghlidi, P.; Cheraghi, M.; Lorestani, B.; Sobhanardakani, S.; Merrikhpour, H. Analysis, spatial distribution and ecological risk assessment of arsenic and some heavy metals of agricultural soils, case study: South of Iran. J. Environ. Health Sci. Eng. 2020, 18, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.S.; Zheng, C.J.; Chen, J.S.; Wu, Y.Y. Study on the background contents on 61 elements of soils in China. Environ. Sci. 1991, 12, 12–19. (In Chinese) [Google Scholar] [CrossRef]
- Qi, Y.C.; Zhang, Q.; Wei, Q.; Huang, R.; Feng, Q.; Wang, J. Study of heavy metal Cd distribution and bio-availability in soil of vegetal bases in Yan’an. Shaanxi J. Agric. Sci. 2020, 66, 41–43. (In Chinese) [Google Scholar]
- Li, Y.; Wang, C.; Yan, C.; Liu, S.; Chen, X.; Zeng, M.; Dong, Y.; Jiao, R. Heavy Metal Concentrations and Accumulation Characteristics of Dominant Woody Plants in Iron and Lead−Zinc Tailing Areas in Jiangxi, Southeast China. Forests 2023, 14, 846. [Google Scholar] [CrossRef]
- Wang, J.X.; Sun, X.C.; Xing, Y.; Xia, J.C.; Feng, X.B. Immobilization of mercury and arsenic in a mine tailing from a typical Carlin-type gold mining site in southwestern part of China. J. Clean. Prod. 2019, 240, 118171. [Google Scholar] [CrossRef]
- Cristaldi, A.; Conti, G.O.; Cosentino, S.L.; Mauromicale, G.; Copat, C.; Grasso, A.; Zuccarello, P.; Fiore, M.; Restuccia, C.; Ferrante, M. Phytoremediation potential of Arundo donax (Giant Reed) in contaminated soil by heavy metals. Environ. Res. 2020, 185, 109427. [Google Scholar] [CrossRef]
- Mao, C.P.; Song, Y.X.; Chen, L.X.; Ji, J.F.; Li, J.Z.; Yuan, X.Y.; Yang, Z.F.; Ayoko, G.A.; Frost, R.L.; Theiss, F. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena 2019, 175, 339–348. [Google Scholar] [CrossRef]
- Ba, Z.D.; Wang, J.F.; Song, C.W.; Du, H.S. Spatial heterogeneity of soil nutrients in black soil areas of Northeast China. Agron. J. 2022, 114, 2021–2026. [Google Scholar] [CrossRef]
- Matschullat, J. Arsenic in the geosphere—A review. Sci. Total Environ. 2000, 249, 297–312. [Google Scholar] [CrossRef]
- Coelho, C.; Foret, C.; Bazin, C.; Leduc, L.; Hammada, M.; Inácio, M.; Bedell, J.P. Bioavailability and bioaccumulation of heavy metals of several soils and sediments (from industrialized urban areas) for Eisenia fetida. Sci. Total Environ. 2018, 635, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Q.; Gao, S.F.; Wang, W.L.; Staunton, S.; Wang, G. Soil arsenic availability and the transfer of soil arsenic to crops in suburban areas in Fujian Province, southeast China. Sci. Total Environ. 2006, 368, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Wei, Y.; Zhao, L.; Zhang, J.; Shangguan, Y.X.; Li, F.S.; Hou, H. Bioaccessibility of antimony and arsenic in highly polluted soils of the mine area and health risk assessment associated with oral ingestion exposure. Ecotoxicol. Environ. Saf. 2014, 110, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Srivastava, P.K. Bioavailability of arsenic in agricultural soils under the influence of different soil properties. SN Appl. Sci. 2020, 2, 153. [Google Scholar] [CrossRef]
- Lin, C.F.; Wu, C.H.; Lai, H.T. Dissolved organic matter and arsenic removal with coupled chitosan/UF operation. Sep. Purif. Technol. 2008, 60, 292–298. [Google Scholar] [CrossRef]
- Chen, Z.L.; An, L.H.; Wei, H.; Zhang, J.Q.; Zou, Q.; Sun, M.Q.; Huang, L.; Liu, M. Nitrate alleviate dissimilatory iron reduction and arsenic mobilization by driving microbial community structure change. Surf. Interfaces 2021, 26, 101421. [Google Scholar] [CrossRef]
- Liu, L.; Shen, R.L.; Zhao, Z.Q.; Ding, L.J.; Cui, H.L.; Li, G.; Yang, Y.P.; Duan, G.L.; Zhu, Y.G. How different nitrogen fertilizers affect arsenic mobility in paddy soil after straw incorporation? J. Hazard. Mater. 2022, 436, 129135. [Google Scholar] [CrossRef]
- Jiang, W.; Hou, Q.; Yang, Z.F.; Zhong, C.; Zheng, G.D.; Yang, Z.Q.; Li, J. Evaluation of potential effects of soil available phosphorus on soil arsenic availability and paddy rice inorganic arsenic content. Environ. Pollut. 2014, 188, 159–165. [Google Scholar] [CrossRef]
- Meharg, A.A.; Hartley-Whitaker, J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 2002, 154, 29–43. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Dai, Y.C.; Lv, J.L.; Liu, K.; Zhao, X.Y.; Cao, Y.F. Major controlling factors and prediction models for arsenic uptake from soil to wheat plants. Ecotoxicol. Environ. Saf. 2016, 130, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Miao, A.J.; Wang, N.X.; Li, C.J.; Sha, J.; Jia, J.B.; Alessi, D.S.; Yan, B.; Ok, Y.S. Arsenic bioaccumulation and biotransformation in aquatic organisms. Environ. Int. 2022, 163, 107221. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Wang, X.Q.; Li, F.B.; Li, B.; Liu, C.P.; Wang, Q.; Lei, J. Arsenic mobility and bioavailability in paddy soil under iron compound amendments at different growth stages of rice. Environ. Pollut. 2017, 224, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Liu, G.N.; Wang, J.; Zhang, E.X.; Hou, J.; Liu, X.H. Heavy metal speciation and risk assessment in dry land and paddy soils near mining areas at Southern China. Environ. Sci. Pollut. Res. 2016, 23, 8709–8720. [Google Scholar] [CrossRef]
- Haque, K.S.; Eberbach, P.L.; Weston, L.A.; Dyall-Smith, M.; Howitt, J.A. Pore Mn2+ dynamics of the rhizosphere of flooded and non-flooded rice during a long wet and drying phase in two rice growing soils. Chemosphere 2015, 134, 16–24. [Google Scholar] [CrossRef]
- Choi, J.; Lee, E.; Choi, S.Q.; Lee, S.; Han, Y.; Kim, H. Arsenic removal from contaminated soils for recycling via oil agglomerate flotation. Chem. Eng. J. 2016, 285, 207–217. [Google Scholar] [CrossRef]
- Xiang, D.F.; Liao, S.J.; Tu, S.X.; Zhu, D.W.; Xie, T.; Wang, G.J. Surfactants enhanced soil arsenic phytoextraction efficiency by Pteris vittata L. Bull. Environ. Contam. Toxicol. 2020, 104, 259–264. [Google Scholar] [CrossRef]






| Index | Max (mg·kg−1) | Min (mg·kg−1) | Mean (mg·kg−1) | Standard Deviation | Coefficient of Variation (%) |
|---|---|---|---|---|---|
| pH | 8.87 | 7.53 | 8.41 | 0.26 | 3.09 |
| SWC (%) | 40.81 | 4.01 | 15.02 | 0.11 | 73.33 |
| SBD (g·cm−3) | 1.81 | 1.03 | 1.33 | 0.19 | 14.29 |
| SOM (g·kg−1) | 5.18 | 0.03 | 1.49 | 1.31 | 87.92 |
| STN (g·kg−1) | 3.58 | 0.42 | 1.25 | 0.80 | 64.00 |
| SAN (mg·kg−1) | 200.60 | 20.04 | 65.12 | 42.51 | 65.28 |
| STP(g·kg−1) | 0.10 | 0.02 | 0.04 | 0.02 | 50.00 |
| SAP (mg·kg-1) | 24.97 | 3.05 | 10.77 | 6.47 | 60.07 |
| STK (g·kg−1) | 29.50 | 10.51 | 21.24 | 4.68 | 22.03 |
| SAK (mg·kg−1) | 232.09 | 23.44 | 72.88 | 54.41 | 74.66 |
| As Characteristics | Min (mg·kg−1) | Max (mg·kg−1) | Mean (mg·kg−1) | Coefficient of Variation (%) | Percentage (%) | Mean Percentage (%) |
|---|---|---|---|---|---|---|
| Mild acidosoluble As | 0.06 | 9.13 | 1.85 | 117.03 | 0.01–0.52 | 0.17 |
| Reducible As | 0.38 | 721.89 | 238.30 | 87.25 | 0.60–53.72 | 20.84 |
| Oxidizable As | 0.02 | 10.15 | 3.44 | 83.79 | 0.03–0.72 | 0.29 |
| Residual As | 8.48 | 1472.57 | 422.92 | 93.84 | 3.87–80.65 | 42.78 |
| Water-soluble As | 4.59 | 1024.77 | 347.90 | 82.70 | 1.58–79.85 | 35.92 |
| Total | 22.08 | 1971.54 | 1034.41 | 63.22 | – | – |
| Plant Species | BCF | TF |
|---|---|---|
| Typha orientalis C. Presl | 0.037 | 0.126 |
| Lythrum salicaria L. | 0.447 | 2.523 |
| Oenanthe javanica (Blume) DC. | 0.042 | 0.233 |
| Equisetum ramosissimum Desf. | 0.263 | 1.145 |
| Phragmites australis (Cav.) Trin. ex Steud. | 0.119 | 2.203 |
| Imperata cylindrica (L.) P. Beauv. | 0.035 | 0.101 |
| Eleusine indica (L.) Gaertn. | 0.034 | 0.624 |
| Setaria viridis (L.) P. Beauv. | 0.015 | 1.068 |
| Bothriochloa ischaemum (L.) Keng | 0.009 | 0.522 |
| Sophora davidii Kom. ex Pavol | 0.047 | 0.533 |
| Melilotus officinalis (L.) Pall. | 0.023 | 0.428 |
| Sonchus wightianus DC. | 0.009 | 2.637 |
| Conyza canadensis (L.) Cronq. | 0.009 | 1.048 |
| Erigeron annuus (L.) Pers. | 0.016 | 0.571 |
| Periploca sepium Bunge | 0.006 | 1.918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Zhai, Y.; Chen, R.; Fan, Y.; Liu, Z.; Zhao, Y.; Li, R.; Xia, L. Characteristics of Soil Arsenic Contamination and the Potential of Pioneer Plants for Arsenic Remediation in Gold Mine Tailings. Toxics 2023, 11, 1025. https://doi.org/10.3390/toxics11121025
Han L, Zhai Y, Chen R, Fan Y, Liu Z, Zhao Y, Li R, Xia L. Characteristics of Soil Arsenic Contamination and the Potential of Pioneer Plants for Arsenic Remediation in Gold Mine Tailings. Toxics. 2023; 11(12):1025. https://doi.org/10.3390/toxics11121025
Chicago/Turabian StyleHan, Lei, Yunmeng Zhai, Rui Chen, Yamin Fan, Zhao Liu, Yonghua Zhao, Risheng Li, and Longfei Xia. 2023. "Characteristics of Soil Arsenic Contamination and the Potential of Pioneer Plants for Arsenic Remediation in Gold Mine Tailings" Toxics 11, no. 12: 1025. https://doi.org/10.3390/toxics11121025