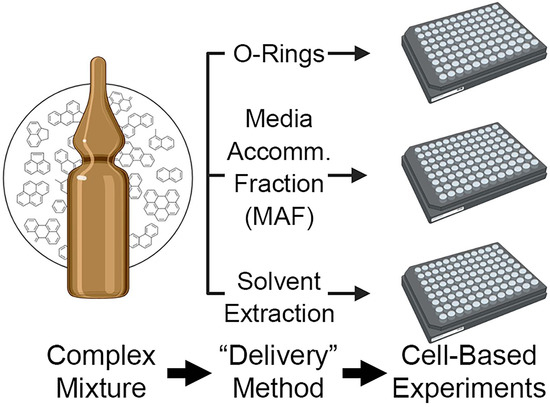
Dosing Methods to Enable Cell-Based In Vitro Testing of Complex Substances: A Case Study with a PAH Mixture
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Biologicals
2.2. Analytical Instrumentation, Method, and Analysis
2.3. Preparing Micro-O-Rings
2.4. Preparing Media Accommodated Fraction (MAF)
2.5. Preparing DMSO Extracts
2.6. Testing Loading Kinetics of Micro-O-Rings
2.7. Assessing Recovery over Time from Exposure to Cell Culture Media without Cells
2.8. Assessing Protein Binding Behavior from Exposure to Cell Culture Media without Cells
2.9. Protein Binding Analysis of the Media-Dissolved Fraction and Unbound Fraction
2.10. In Vitro Experiments
2.11. Quantification of Media-Dissolved Fraction from In Vitro Samples
3. Results and Discussion
3.1. Micro-O-Ring Loading of PAH
3.2. Testing of Dosing Methods in Complete Media without Cells: Analysis of Recovery from Cell Culture Media over Time
3.3. Testing of Dosing Methods in Complete Media without Cells: Protein Binding Analysis of Media-Dissolved and Unbound Fractions
3.4. Application of Each Dosing Method to In Vitro Studies: Determination of Media-Dissolved Fraction after Incubation In Vitro
3.5. Application of Each Dosing Method to In Vitro Studies: Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Knap, A.H.; Rusyn, I. Environmental exposures due to natural disasters. Rev. Environ. Health 2016, 31, 89–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Scientific Committee; More, S.J.; Bampidis, V.; Benford, D.; Bennekou, S.H.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Koutsoumanis, K.; Naegeli, H. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 2019, 17, e05634. [Google Scholar] [PubMed] [Green Version]
- Hodson, P.V.; Adams, J.; Brown, R.S. Oil toxicity test methods must be improved. Environ. Toxicol. Chem. 2019, 38, 302–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauderly, J.L. Toxicological approaches to complex mixtures. Environ. Health Perspect. 1993, 101 (Suppl. 4), 155–165. [Google Scholar]
- Kortenkamp, A.; Faust, M. Regulate to reduce chemical mixture risk. Science 2018, 361, 224–226. [Google Scholar] [CrossRef] [Green Version]
- Meek, M.; Boobis, A.R.; Crofton, K.M.; Heinemeyer, G.; Van Raaij, M.; Vickers, C. Risk assessment of combined exposure to multiple chemicals: A who/ipcs framework. Regul. Toxicol. Pharmacol. 2011, 60, S1–S14. [Google Scholar]
- Escher, B.I.; Stapleton, H.M.; Schymanski, E.L. Tracking complex mixtures of chemicals in our changing environment. Science 2020, 367, 388–392. [Google Scholar] [CrossRef]
- Salvito, D.; Fernandez, M.; Jenner, K.; Lyon, D.Y.; de Knecht, J.; Mayer, P.; MacLeod, M.; Eisenreich, K.; Leonards, P.; Cesnaitis, R.; et al. Improving the environmental risk assessment of substances of unknown or variable composition, complex reaction products, or biological materials. Environ. Toxicol. Chem. 2020, 39, 2097–2108. [Google Scholar] [CrossRef]
- Drakvik, E.; Altenburger, R.; Aoki, Y.; Backhaus, T.; Bahadori, T.; Barouki, R.; Brack, W.; Cronin, M.T.D.; Demeneix, B.; Hougaard Bennekou, S.; et al. Statement on advancing the assessment of chemical mixtures and their risks for human health and the environment. Environ. Int. 2020, 134, 105267. [Google Scholar] [CrossRef]
- Hsieh, N.H.; Chen, Z.; Rusyn, I.; Chiu, W.A. Risk characterization and probabilistic concentration-response modeling of complex environmental mixtures using new approach methodologies (nams) data from organotypic in vitro human stem cell assays. Environ. Health Perspect. 2021, 129, 17004. [Google Scholar] [CrossRef]
- Chen, Z.; Lloyd, D.; Zhou, Y.H.; Chiu, W.A.; Wright, F.A.; Rusyn, I. Risk characterization of environmental samples using in vitro bioactivity and polycyclic aromatic hydrocarbon concentrations data. Toxicol. Sci. 2021, 179, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Ford, L.C.; Rusyn, I.; Chiu, W.A. Cumulative risk meets inter-individual variability: Probabilistic concentration addition of complex mixture exposures in a population-based human in vitro model. Toxics 2022, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.S.; Chen, Z.; Hsieh, N.H.; Lin, T.E. Chemical and biological assessments of environmental mixtures: A review of current trends, advances, and future perspectives. J. Hazard. Mater. 2022, 432, 128658. [Google Scholar] [CrossRef] [PubMed]
- CONCAWE. The Use of the Dimethyl Sulphoxide (Dmso) Extract by the ip 346 Method as an Indicator of the Carcinogenicity of Lubricant Base Oils and Distillate Aromatic Extracts; CONCAWE: Brussels, Belgium, 1994. [Google Scholar]
- Grimm, F.A.; Iwata, Y.; Sirenko, O.; Chappell, G.A.; Wright, F.A.; Reif, D.M.; Braisted, J.; Gerhold, D.L.; Yeakley, J.M.; Shepard, P.; et al. A chemical-biological similarity-based grouping of complex substances as a prototype approach for evaluating chemical alternatives. Green Chem. 2016, 18, 4407–4419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.S.; Ferguson, K.C.; Rusyn, I.; Chiu, W.A. In vitro bioavailability of the hydrocarbon fractions of dimethyl sulfoxide extracts of petroleum substances. Toxicol. Sci. 2020, 174, 168–177. [Google Scholar] [CrossRef]
- Wheeler, J.R.; Lyon, D.; Di Paolo, C.; Grosso, A.; Crane, M. Challenges in the regulatory use of water-accommodated fractions for assessing complex substances. Environ. Sci. Eur. 2020, 32, 153. [Google Scholar] [CrossRef]
- House, J.S.; Grimm, F.A.; Klaren, W.D.; Dalzell, A.; Kuchi, S.; Zhang, S.D.; Lenz, K.; Boogaard, P.J.; Ketelslegers, H.B.; Gant, T.W.; et al. Grouping of uvcb substances with new approach methodologies (nams) data. ALTEX 2021, 38, 123–137. [Google Scholar] [CrossRef]
- ECHA. Testing Proposal Decision on Substance ec 295-332-8 "Extracts (Petroleum), Deasphalted Vacuum Residue Solvent"; European Chemicals Agency: Helsinki, Finland, 2020. [Google Scholar]
- OECD. Guidance Document on Aqueous-Phase Aquatic Toxicity Testing of Difficult Test Chemicals; OECD: Paris, France, 2019. [Google Scholar]
- Hammershoj, R.; Birch, H.; Sjoholm, K.K.; Mayer, P. Accelerated passive dosing of hydrophobic complex mixtures-controlling the level and composition in aquatic tests. Environ. Sci. Technol. 2020, 54, 4974–4983. [Google Scholar] [CrossRef]
- Niehus, N.C.; Floeter, C.; Hollert, H.; Witt, G. Miniaturised marine algae test with polycyclic aromatic hydrocarbons—Comparing equilibrium passive dosing and nominal spiking. Aquat. Toxicol. 2018, 198, 190–197. [Google Scholar] [CrossRef]
- Philibert, D.; Parkerton, T.; Marteinson, S.; de Jourdan, B. Assessing the toxicity of individual aromatic compounds and mixtures to american lobster (homarus americanus) larvae using a passive dosing system. Environ. Toxicol. Chem 2021, 40, 1379–1388. [Google Scholar] [CrossRef]
- Smith, K.E.; Oostingh, G.J.; Mayer, P. Passive dosing for producing defined and constant exposure of hydrophobic organic compounds during in vitro toxicity tests. Chem. Res. Toxicol. 2010, 23, 55–65. [Google Scholar] [CrossRef]
- Trac, L.N.; Sjo Holm, K.K.; Birch, H.; Mayer, P. Passive dosing of petroleum and essential oil uvcbs-whole mixture toxicity testing at controlled exposure. Environ. Sci. Technol. 2021, 55, 6150–6159. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.M.; Aurand, D.; Bragin, G.E.; Clark, J.R.; Coelho, G.M.; Sowby, M.L.; Tjeerdema, R.S. Standardization of the preparation and quantitation of water-accommodated fractions of petroleum for toxicity testing. Mar. Pollut. Bull. 2000, 40, 1007–1016. [Google Scholar] [CrossRef]
- Bera, G.; Parkerton, T.; Redman, A.; Turner, N.R.; Renegar, D.A.; Sericano, J.L.; Knap, A.H. Passive dosing yields dissolved aqueous exposures of crude oil comparable to the croserf (chemical response to oil spill: Ecological effects research forum) water accommodated fraction method. Environ. Toxicol. Chem. 2018, 37, 2810–2819. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.A.; Parkerton, T.F.; Redman, A.D.; Lewis, C.; Galloway, T.S. Miniaturised marine tests as indicators of aromatic hydrocarbon toxicity: Potential applicability to oil spill assessment. Mar. Pollut. Bull. 2021, 165, 112151. [Google Scholar] [CrossRef]
- Heger, S.; Bluhm, K.; Brendt, J.; Mayer, P.; Anders, N.; Schaffer, A.; Seiler, T.B.; Hollert, H. Microscale in vitro assays for the investigation of neutral red retention and ethoxyresorufin-o-deethylase of biofuels and fossil fuels. PLoS ONE 2016, 11, e0163862. [Google Scholar] [CrossRef] [Green Version]
- Parkerton, T.F.; Letinski, D.J.; Febbo, E.J.; Butler, J.D.; Sutherland, C.A.; Bragin, G.E.; Hedgpeth, B.M.; Kelley, B.A.; Redman, A.D.; Mayer, P.; et al. Assessing toxicity of hydrophobic aliphatic and monoaromatic hydrocarbons at the solubility limit using novel dosing methods. Chemosphere 2021, 265, 129174. [Google Scholar] [CrossRef]
- Rojo-Nieto, E.; Smith, K.E.C.; Perales, J.A.; Mayer, P. Recreating the seawater mixture composition of hocs in toxicity tests with artemia franciscana by passive dosing. Aquat. Toxicol. 2012, 120, 27–34. [Google Scholar] [CrossRef]
- Turner, N.R.; Bera, G.; Renegar, D.A.; Frank, T.M.; Riegl, B.M.; Sericano, J.L.; Sweet, S.; Knap, A.H. Measured and predicted acute toxicity of phenanthrene and mc252 crude oil to vertically migrating deep-sea crustaceans. Environ. Sci. Pollut. Res. Int. 2020, 27, 45270–45281. [Google Scholar] [CrossRef]
- Redman, A.D.; Parkerton, T.F. Guidance for improving comparability and relevance of oil toxicity tests. Mar. Pollut. Bull. 2015, 98, 156–170. [Google Scholar] [CrossRef]
- Henneberger, L.; Muhlenbrink, M.; Heinrich, D.J.; Teixeira, A.; Nicol, B.; Escher, B.I. Experimental validation of mass balance models for in vitro cell-based bioassays. Environ. Sci. Technol. 2020, 54, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Proenca, S.; Escher, B.I.; Fischer, F.C.; Fisher, C.; Gregoire, S.; Hewitt, N.J.; Nicol, B.; Paini, A.; Kramer, N.I. Effective exposure of chemicals in in vitro cell systems: A review of chemical distribution models. Toxicol. Vitro 2021, 73, 105133. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S. Benzene Oral Bioavailability Assessment Using In Vitro Digestion Model in Combination with Cell Culture Methodology. Ph.D. Thesis, University of Saskatchewan Saskatoon, Saskatoon, SK, Canada, 2021. [Google Scholar]
- Chapman, F.M.; Sparham, C.; Hastie, C.; Sanders, D.J.; van Egmond, R.; Chapman, K.E.; Doak, S.H.; Scott, A.D.; Jenkins, G.J.S. Comparison of passive-dosed and solvent spiked exposures of pro-carcinogen, benzo[a]pyrene, to human lymphoblastoid cell line, mcl-5. Toxicol. Vitro 2020, 67, 104905. [Google Scholar] [CrossRef] [PubMed]
- Oostingh, G.J.; Smith, K.E.C.; Tischler, U.; Radauer-Preiml, I.; Mayer, P. Differential immunomodulatory responses to nine polycyclic aromatic hydrocarbons applied by passive dosing. Toxicol. Vitro 2015, 29, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Johann, S.; Gossen, M.; Behnisch, P.A.; Hollert, H.; Seiler, T.B. Combining different in vitro bioassays to evaluate genotoxicity of water-accommodated fractions from petroleum products. Toxics 2020, 8, 45. [Google Scholar] [CrossRef]
- Nakai, D.; Kumamoto, K.; Sakikawa, C.; Kosaka, T.; Tokui, T. Evaluation of the protein binding ratio of drugs by a micro-scale ultracentrifugation method. J. Pharm. Sci. 2004, 93, 847–854. [Google Scholar] [CrossRef]
- Stout, S.A.; Wang, Z. Standard Handbook Oil Spill Environmental Forensics: Fingerprinting and Source Identification, 2nd ed.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- IARC. Chemical agents and related occupations—A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100F, 1–567. [Google Scholar]
- Moreno Frias, M.; Jimenez Torres, M.; Garrido Frenich, A.; Martinez Vidal, J.L.; Olea-Serrano, F.; Olea, N. Determination of organochlorine compounds in human biological samples by gc-ms/ms. Biomed. Chromatogr. 2004, 18, 102–111. [Google Scholar] [CrossRef]
- Quimby, B.D.; Andrianova, A.A. Optimized gc/ms Analysis for Pahs in Challenging Matrices; Environmental, Food Testing and Agriculture; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2019. [Google Scholar]
- Stibany, F.; Ewald, F.; Miller, I.; Hollert, H.; Schaffer, A. Improving the reliability of aquatic toxicity testing of hydrophobic chemicals via equilibrium passive dosing—A multiple trophic level case study on bromochlorophene. Sci. Total Environ. 2017, 584–585, 96–104. [Google Scholar] [CrossRef]
- Fischer, F.C.; Henneberger, L.; Schlichting, R.; Escher, B.I. How to improve the dosing of chemicals in high-throughput in vitro mammalian cell assays. Chem. Res. Toxicol. 2019, 32, 1462–1468. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Determining Carcinogenic Potential of Virgin Base Oils in Metalworking Fluids; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Grimm, F.A.; Iwata, Y.; Sirenko, O.; Bittner, M.; Rusyn, I. High-content assay multiplexing for toxicity screening in induced pluripotent stem cell-derived cardiomyocytes and hepatocytes. Assay Drug Dev. Technol. 2015, 13, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Kamalian, L.; Chadwick, A.E.; Bayliss, M.; French, N.S.; Monshouwer, M.; Snoeys, J.; Park, B.K. The utility of hepg2 cells to identify direct mitochondrial dysfunction in the absence of cell death. Toxicol. In Vitro 2015, 29, 732–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, L.C.; Jang, S.; Chen, Z.; Zhou, Y.H.; Gallins, P.J.; Wright, F.A.; Chiu, W.A.; Rusyn, I. A population-based human in vitro approach to quantify in-ter-individual variability in responses to chemical mixtures. Toxics 2022, 10, 441. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Nguyen, H.T.L.; Truong, K.D. Comparative cytotoxic effects of methanol, ethanol and dmso on human cancer cell lines. Biomed. Res. Ther. 2020, 7, 3855–3859. [Google Scholar] [CrossRef]
- Grant, S.; Schacht, V.J.; Escher, B.I.; Hawker, D.W.; Gaus, C. Experimental solubility approach to determine pdms-water partition constants and pdms activity coefficients. Environ. Sci. Technol. 2016, 50, 3047–3054. [Google Scholar] [CrossRef]
- Valdiviezo, A.; Luo, Y.S.; Chen, Z.; Chiu, W.A.; Rusyn, I. Quantitative in vitro-to-in vivo extrapolation for mixtures: A case study of superfund priority list pesticides. Toxicol. Sci. 2021, 183, 60–69. [Google Scholar] [CrossRef]
- Fischer, F.C.; Cirpka, O.A.; Goss, K.U.; Henneberger, L.; Escher, B.I. Application of experimental polystyrene partition constants and diffusion coefficients to predict the sorption of neutral organic chemicals to multiwell plates in in vivo and in vitro bioassays. Environ. Sci. Technol. 2018, 52, 13511–13522. [Google Scholar] [CrossRef]
- Kramer, N.I.; Busser, F.J.M.; Oosterwijk, M.T.T.; Schirmer, K.; Escher, B.I.; Hermens, J.L.M. Development of a partition-controlled dosing system for cell assays. Chem. Res. Toxicol. 2010, 23, 1806–1814. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.N.; Holmstrup, M.; Smith, K.E.C.; Mayer, P. Passive dosing of polycyclic aromatic hydrocarbon (pah) mixtures to terrestrial springtails: Linking mixture toxicity to chemical activities, equilibrium lipid concentrations, and toxic units. Environ. Sci. Technol. 2013, 47, 7020–7027. [Google Scholar] [CrossRef]
- Smith, K.E.C.; Dom, N.; Blust, R.; Mayer, P. Controlling and maintaining exposure of hydrophobic organic compounds in aquatic toxicity tests by passive dosing. Aquat. Toxicol. 2010, 98, 15–24. [Google Scholar] [CrossRef]
- Stibany, F.; Schmidt, S.N.; Schaffer, A.; Mayer, P. Aquatic toxicity testing of liquid hydrophobic chemicals—Passive dosing exactly at the saturation limit. Chemosphere 2017, 167, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Stibany, F.; Schmidt, S.N.; Mayer, P.; Schaffer, A. Toxicity of dodecylbenzene to algae, crustacean, and fish—Passive dosing of highly hydrophobic liquids at the solubility limit. Chemosphere 2020, 251, 126396. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Sardana, M.K.; Borenfreund, E. Acute cytotoxicities of polynuclear aromatic hydrocarbons determined in vitro with the human liver tumor cell line, hepg2. Cell Biol. Toxicol. 1988, 4, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Pant, A.B.; Das, M. In vitro cytotoxicity of polycyclic aromatic hydrocarbon residues arising through repeated fish fried oil in human hepatoma hep g2 cell line. Toxicol. In Vitro 2006, 20, 308–316. [Google Scholar] [CrossRef]
- Shukla, H.; Lee, H.Y.; Koucheki, A.; Bibi, H.A.; Gaje, G.; Sun, X.; Zhu, H.; Li, Y.R.; Jia, Z. Targeting glutathione with the triterpenoid cddo-im protects against benzo-a-pyrene-1,6-quinone-induced cytotoxicity in endothelial cells. Mol. Cell Biochem. 2020, 474, 27–39. [Google Scholar] [CrossRef]
- Wetmore, B.A. Quantitative in vitro-to-in vivo extrapolation in a high-throughput environment. Toxicology 2015, 332, 94–101. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordova, A.C.; Ford, L.C.; Valdiviezo, A.; Roman-Hubers, A.T.; McDonald, T.J.; Chiu, W.A.; Rusyn, I. Dosing Methods to Enable Cell-Based In Vitro Testing of Complex Substances: A Case Study with a PAH Mixture. Toxics 2023, 11, 19. https://doi.org/10.3390/toxics11010019
Cordova AC, Ford LC, Valdiviezo A, Roman-Hubers AT, McDonald TJ, Chiu WA, Rusyn I. Dosing Methods to Enable Cell-Based In Vitro Testing of Complex Substances: A Case Study with a PAH Mixture. Toxics. 2023; 11(1):19. https://doi.org/10.3390/toxics11010019
Chicago/Turabian StyleCordova, Alexandra C., Lucie C. Ford, Alan Valdiviezo, Alina T. Roman-Hubers, Thomas J. McDonald, Weihsueh A. Chiu, and Ivan Rusyn. 2023. "Dosing Methods to Enable Cell-Based In Vitro Testing of Complex Substances: A Case Study with a PAH Mixture" Toxics 11, no. 1: 19. https://doi.org/10.3390/toxics11010019