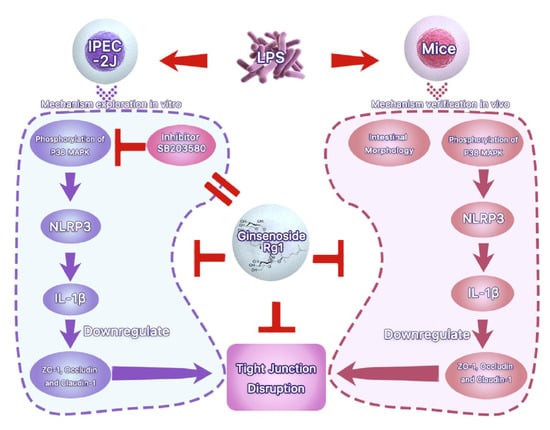
Ginsenoside Rg1 Mitigates Porcine Intestinal Tight Junction Disruptions Induced by LPS through the p38 MAPK/NLRP3 Inflammasome Pathway
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Effects of Different Concentrations of LPS on ZO-1, Occludin and Claudin-1 Expression in IPEC-J2 Cells
3.2. Rg1 Inhibited LPS-Induced Morphological Damage of Ipec-j2 Cells via Downregulating ZO-1, Occludin and Claudin-1 Expression
3.3. Rg1 Inhibits LPS-Induced Tight Junction Disruption in IPEC-J2 Cells by Interfering with P38 MAPK Signaling Pathway
3.4. Rg1 Inhibits the mRNA and Protein Expression of NLRP3 and IL-1β through p38 MAPK Signaling Pathway
3.5. Effects of MCC950 and IL-1Ra on Expression of ZO-1, Occludin, and Claudin-1 in LPS-Treated IPEC-J2 Cells
3.6. Rg1 Inhibits Tight Junction Disruption via P38 MAPK Signaling in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, D.; Liu, W.; Hou, Y.; Wang, L.; Zhao, D.; Wu, T.; Ding, B.; Wu, G. Establishment of a porcine model of indomethacin-induced intestinal injury. Front. Biosci. 2018, 23, 2166–2176. [Google Scholar]
- Suzuki, T.; Hara, H. Role of flavonoids in intestinal tight junction regulation. J. Nutr. Biochem. 2011, 22, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Q.; Wang, Y.; Liang, T.; Li, X.; Wang, D.; Wang, X.; Zhu, H.; Xiao, K. Necroptosis is active and contributes to intestinal injury in a piglet model with lipopolysaccharide challenge. Cell Death Dis. 2021, 12, 62. [Google Scholar] [CrossRef]
- Prima, V.; Wang, A.; Molina, G.; Wang, K.; Svetlov, S.I. Inhibition of LPS toxicity by hepatic argininosuccinate synthase (ASS): Novel roles for ASS in innate immune responses to bacterial infection. Int. Immunopharmacol. 2011, 11, 1180–1188. [Google Scholar] [CrossRef]
- Tsai, H.C.; Chen, H.; Mochly-Rosen, D.; Li, Y.E.; Chen, M.H. The Role of Alcohol, LPS Toxicity, and ALDH2 in Dental Bony Defects. Biomolecules 2021, 11, 651. [Google Scholar] [CrossRef]
- Gonzalo, S.; Grasa, L.; Arruebo, M.P.; Plaza, M.A.; Murillo, M.D. Inhibition of p38 MAPK improves intestinal disturbances and oxidative stress induced in a rabbit endotoxemia model. Neurogastroenterol. Motil. 2010, 22, 564. [Google Scholar] [CrossRef]
- Hsu, J.T.; Le, P.H.; Lin, C.J.; Chen, T.H.; Kuo, C.J.; Chiang, K.C.; Yeh, T.S. Mechanism of salutary effects of melatonin-mediated liver protection after trauma-hemorrhage: p38 MAPK-dependent iNOS/HIF-1α pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G427–G433. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, F.; Wang, L.; Gao, M.; Xie, Y.; Sun, Y.; Liu, H.; Yuan, Y.; Yi, W.; Huang, Z.; et al. Virus-induced p38 MAPK activation facilitates viral infection. Theranostics 2020, 10, 12223–12240. [Google Scholar] [CrossRef]
- Ono, K.; Han, J. The p38 signal transduction pathway: Activation and function. Cell. Signal. 2000, 12, 1–13. [Google Scholar] [CrossRef]
- Liu, X.M.; Chen, Q.H.; Hu, Q.; Liu, Z.; Wu, Q.; Liang, S.S.; Zhang, H.G.; Zhang, Q.; Zhang, X.K. Dexmedetomidine protects intestinal ischemia-reperfusion injury via inhibiting p38 MAPK cascades. Exp. Mol. Pathol. 2020, 115, 104444. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yin, L.; Li, J.Y.; Li, Q.; Shi, D.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, Y.; et al. Glutamate attenuates lipopolysaccharide-induced oxidative damage and mRNA expression changes of tight junction and defensin proteins, inflammatory and apoptosis response signaling molecules in the intestine of fish. Fish Shellfish Immunol. 2017, 70, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.Y.; Tu, C.E.; Wang, S.C.; Hung, Y.L.; Su, C.C.; Fang, S.H.; Chen, C.S.; Liu, P.L.; Cheng, W.C.; Huang, Y.W.; et al. Corylin inhibits LPS-induced inflammatory response and attenuates the activation of NLRP3 inflammasome in microglia. BMC Complement. Altern. Med. 2018, 18, 221. [Google Scholar] [CrossRef]
- Utech, M.; Mennigen, R.; Bruewer, M. Endocytosis and recycling of tight junction proteins in inflammation. J. Biomed. Biotechnol. 2010, 2010, 484987. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng. Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, J.; Wang, J.; Li, X.; Li, J.; Chu, S.; Li, L.; Chen, N.; Zhang, L. Ginsenoside Rg1 prevent and treat inflammatory diseases: A review. Int. Immunopharmacol. 2020, 87, 106805. [Google Scholar] [CrossRef]
- Xin, Y.; Wei, J.; Chunhua, M.; Danhong, Y.; Jianguo, Z.; Zongqi, C.; Jian-An, B. Protective effects of Ginsenoside Rg1 against carbon tetrachloride-induced liver injury in mice through suppression of inflammation. Phytomedicine 2016, 23, 583–588. [Google Scholar] [CrossRef]
- Li, Q.; Xiang, Y.; Chen, Y.; Tang, Y.; Zhang, Y. Ginsenoside Rg1 Protects Cardiomyocytes Against Hypoxia/Reoxygenation Injury via Activation of Nrf2/HO-1 Signaling and Inhibition of JNK. Cell. Physiol. Biochem. 2017, 44, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Wang, P.; Fan, C.; Zhang, P.; Shen, J.; Yu, S.Y. Ginsenoside-Rg1 Rescues Stress-Induced Depression-Like Behaviors via Suppression of Oxidative Stress and Neural Inflammation in Rats. Oxid. Med. Cell Longev. 2020, 2020, 2325391. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cai, H.A.; Zhang, M.S.; Liao, R.Y.; Huang, X.; Hu, F.D. Ginsenoside Rg1 promoted the wound healing in diabetic foot ulcers via miR-489-3p/Sirt1 axis. J. Pharmacol. Sci. 2021, 147, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Duan, H.; Wang, W.; Zhao, S.; Khan, G.J.; Wang, M.; Zhang, Y.; Thakur, K.; Fang, X.; Wu, C.; et al. Ginsenoside Rg1 ameliorates blood-brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release. Acta Pharm. Sin. B 2021, 11, 3493–3507. [Google Scholar] [CrossRef]
- Yu, L.; Gan, X.; Liu, X.; An, R. Calcium oxalate crystals induces tight junction disruption in distal renal tubular epithelial cells by activating ROS/Akt/p38 MAPK signaling pathway. Ren. Fail. 2017, 39, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Jeong, C.H.; Seok, J.S.; Petriello, M.C.; Han, S.G. Arsenic downregulates tight junction claudin proteins through p38 and NF-κB in intestinal epithelial cell line, HT-29. Toxicology 2017, 379, 31–39. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhai, Y.; Liang, S.; Mori, Y.; Han, R.; Sutterwala, F.S.; Qiao, L. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat. Commun. 2013, 4, 1611. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, C.; Xing, Y.; Xue, G.; Zhang, Q.; Pan, F.; Wu, G.; Hu, Y.; Guo, Q.; Lu, A.; et al. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat. Commun. 2017, 8, 1896. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, V.; Santiago-Moreno, J.; Doré, S. Ginseng: A promising neuroprotective strategy in stroke. Front. Cell. Neurosci. 2015, 8, 457. [Google Scholar] [CrossRef] [Green Version]
- Hong, B.N.; Ji, M.G.; Kang, T.H. The efficacy of red ginseng in type 1 and type 2 diabetes in animals. Evid. Based Complement. Altern. Med. 2013, 2013, 593181. [Google Scholar] [CrossRef]
- Ernst, E. Complementary/alternative medicine for hypertension: A mini-review. Wien Med. Wochenschr. 2005, 155, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Ikenouchi, J.; Katahira-Tayama, S.; Furuse, K.; Sasaki, H.; Nakayama, M.; Matsui, T.; Tsukita, S.; Furuse, M.; Tsukita, S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 2006, 126, 741–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Itallie, C.M.; Holmes, J.; Bridges, A.; Gookin, J.L.; Coccaro, M.R.; Proctor, W.; Colegio, O.R.; Anderson, J.M. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J. Cell Sci. 2008, 121, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Li, H.; Liu, Z.; Li, C.; Chen, Y.; Jiang, C.; Yu, Y.; Tian, Z. Impaired intestinal barrier function in a mouse model of hyperuricemia. Mol. Med. Rep. 2019, 20, 3292–3300. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Deng, J.; Hu, X.; Zhou, S.; Wu, J.; Xiao, D.; Darko, K.O.; Huang, Y.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef]
- Feng, S.; Zou, L.; Wang, H.; He, R.; Liu, K.; Zhu, H. RhoA/ROCK-2 Pathway Inhibition and Tight Junction Protein Upregulation by Catalpol Suppresses Lipopolysaccaride-Induced Disruption of Blood-Brain Barrier Permeability. Molecules 2018, 23, 2371. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Liu, L.; Tao, W.; Pei, X.; Wang, G.; Wang, M. Clostridium Tyrobutyricum Protect Intestinal Barrier Function from LPS-Induced Apoptosis via P38/JNK Signaling Pathway in IPEC-J2 Cells. Cell. Physiol. Biochem. 2018, 46, 1779–1792. [Google Scholar] [CrossRef]
- Jin, J.; Zhong, Y.; Long, J.; Wu, T.; Jiang, Q.; Wang, H.; Ge, W.; Zhao, H.; Liu, D. Ginsenoside Rg1 relieves experimental colitis by regulating balanced differentiation of Tfh/Treg cells. Int. Immunopharmacol. 2021, 100, 108133. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Lu, J.; Jiao, J.; Yang, Y.; Zhao, H.; Liang, Z.; Zheng, H. Ginsenoside Rg1 improves cognitive capability and affects the microbiota of large intestine of tree shrew model for Alzheimer’s disease. Mol. Med. Rep. 2021, 23, 291. [Google Scholar] [CrossRef]
- Long, J.; Liu, X.K.; Kang, Z.P.; Wang, M.X.; Zhao, H.M.; Huang, J.Q.; Xiao, Q.P.; Liu, D.Y.; Zhong, Y.B. Ginsenoside Rg1 ameliorated experimental colitis by regulating the balance of M1/M2 macrophage polarization and the homeostasis of intestinal flora. Eur. J. Pharmacol. 2022, 917, 174742. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xie, K.; Zhang, Y.; Xie, Q.; He, X.; Zhang, H. Effects of Dietary Ginsenoside Rg1 Supplementation on Growth Performance, Gut Health, and Serum Immunity in Broiler Chickens. Front. Nutr. 2021, 8, 705279. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Y.; Fan, Y.M.; Ga, Y.; Zhang, Y.N.; Han, J.C.; Hao, Z.H. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-κB pathway. Phytomedicine 2021, 92, 153743. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.Q.; Gao, F.F.; Wu, W.; Li, C.; Pan, Z.; Sun, J.; Wang, H.; Huang, C.; Lee, S.H.; Quan, J.H.; et al. Expression profiles of NOD-like receptors and regulation of NLRP3 inflammasome activation in Toxoplasma gondii-infected human small intestinal epithelial cells. Parasites Vectors 2021, 14, 153. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Li, L.; Han, Y.; Dong, X.; Yang, L.; Li, X.; Li, W.; Li, W. Ginsenoside Rg1 ameliorates aging-induced liver fibrosis by inhibiting the NOX4/NLRP3 inflammasome in SAMP8 mice. Mol. Med. Rep. 2021, 24, 801. [Google Scholar] [CrossRef]
- Li, T.; Wu, Y.N.; Wang, H.; Ma, J.Y.; Zhai, S.S.; Duan, J. Dapk1 improves inflammation, oxidative stress and autophagy in LPS-induced acute lung injury via p38MAPK/NF-κB signaling pathway. Mol. Immunol. 2020, 120, 13–22. [Google Scholar] [CrossRef]
- Li, D.; Ren, W.; Jiang, Z.; Zhu, L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol. Med. Rep. 2018, 18, 4399–4409. [Google Scholar] [CrossRef] [Green Version]






| Genes | Primer Sequences (5′-3′) | Length (bp) | Accession No. |
|---|---|---|---|
| NLRP3 (Sus) | F: TCCACACTTCTGACTTCTAAC | 241 | NM_001256770.2 |
| R: CCTGCTTCCACCACTACT | |||
| IL-1β (Sus) | F: CCCAAAAGTTACCCGAAGAGG | 125 | NM_214055.1 |
| R: TCTGCTTGAGAGGTGCTGATG | |||
| β-actin (Sus) | F: CTCGATCATGAAGTGCGACGT | 114 | U07786.1 |
| R: GTGATCTCCTTCTGCATCCTGTC | |||
| NLRP3 (Mus) | F: CTGTAACATTCGGAGATTGTGGTT | 73 | XM_006532858.2 |
| R: GACCAAGGAGATGTCGAAGCA | |||
| IL-1β (Mus) | F: GAAGAAGAGCCCATCCTCTG | 98 | NM_008361.4 |
| R: TCATCTCGGAGC CTGTAGTG | |||
| β-actin (Mus) | F: CCCTGGAGAAGAGCTACGAG R: TAGTTTCGTGAATGCCGCAG | 120 | NM_007393.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Zhou, Y.; Zhu, C.; Ren, T.; Zhang, Y.; Xiao, L.; Fang, B. Ginsenoside Rg1 Mitigates Porcine Intestinal Tight Junction Disruptions Induced by LPS through the p38 MAPK/NLRP3 Inflammasome Pathway. Toxics 2022, 10, 285. https://doi.org/10.3390/toxics10060285
Kang J, Zhou Y, Zhu C, Ren T, Zhang Y, Xiao L, Fang B. Ginsenoside Rg1 Mitigates Porcine Intestinal Tight Junction Disruptions Induced by LPS through the p38 MAPK/NLRP3 Inflammasome Pathway. Toxics. 2022; 10(6):285. https://doi.org/10.3390/toxics10060285
Chicago/Turabian StyleKang, Jian, Yanhong Zhou, Chunyang Zhu, Tian Ren, Yong Zhang, Longfei Xiao, and Binghu Fang. 2022. "Ginsenoside Rg1 Mitigates Porcine Intestinal Tight Junction Disruptions Induced by LPS through the p38 MAPK/NLRP3 Inflammasome Pathway" Toxics 10, no. 6: 285. https://doi.org/10.3390/toxics10060285