Composition of Three Common Chinese Herbal Medicines and the Influence of Preparation Types on the Bioaccessibility of Trace Elements
Abstract
:1. Introduction
2. Materials and Methods
2.1. CHM Materials and Analysis of Total Concentration of Trace elements
2.2. Micro-XRF Analysis for the Distribution of Elements and the Inorganic Compositions
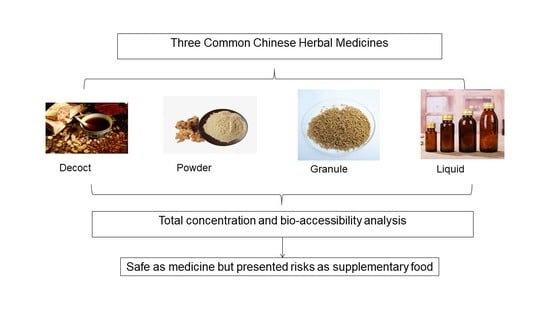
2.3. Preparation Types of CHMs
- (1)
- Decoct: the raw CHM material was placed in a bottle with 678.8 mL of ultrapure water, soaked for 40 min, and then boiled for 3 min once every 40 min. This process was repeated thrice. The volume of the solution was controlled within 200 mL and centrifuged at 5000× g for 5 min.
- (2)
- Powder: the raw CHM material was first ground to powder (<150 μm), and the same procedure for decoction was followed.
- (3)
- Granule: the raw CHM material was soaked and boiled twice using the same procedure as a decoction, and then 95% ethanol was added to the decoction until the ethanol content reached 70%. The supernatant was collected and condensed into a 10 mL extract. The extract was mixed with dextrin, sucrose, and 95% ethanol, dried at 50 °C for 15 min, and granulated into particles with the size of <1.18 mm.
- (4)
- Liquid: the raw CHM was soaked and boiled twice by using the same procedure as decoction and condensed to an extract with a density of 1.08–1.12 g/cm3. The supernatant was collected, added with 95% ethanol until the ethanol content reached 60%, and condensed again to an extract with a density of 1.30–1.33 g/cm3. The supernatant was mixed with sucrose syrup (60%) and diluted to a volume of 1000 mL.
2.4. Extraction and Analysis for the Bioaccessible Fraction of Trace Elements in CHMs
2.5. Chemical Analysis and Quality Control
3. Results
3.1. Total Concentration of Trace Elements in CHMs
3.2. Bioaccessible Fractions of Trace Elements after Different Preparation Ways
3.3. Inorganic Compositions of CHMs and Their Spatial Distribution
4. Discussion
4.1. Potential Health Risks of Trace Elements in CHMs as Dietary Supplements
4.2. The Comparatively Higher Bioaccessibility of as in Isatidis May Result from Sulfur Fumigation
4.3. Preparation Ways for the CHMs
4.4. Appropriate Evaluation Criteria of Trace Elements in CHMs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, H.D.; Ma, Q.Q.; Ye, L.; Piao, G.C. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, C.-Q.; Yue, X.-Q.; Ling, C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J. Integr. Med. 2014, 12, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, M. Traditional Chinese Medicine Intervenes in Vascular Dementia: Traditional Medicine Brings New Expectations. Front. Pharmacol. 2021, 12, 689625. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Long, S.Q.; Deng, Z.Y.; Wu, W.Y. Positive Role of Chinese Herbal Medicine in Cancer Immune Regulation. Am. J. Chin. Med. 2020, 48, 1577–1592. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lin, L.; Ni, Q.; Su, C.L. Chinese Medicine for Treating Diabetic Nephropathy. Chin. J. Integr. Med. 2011, 17, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Li, M.G.; Wang, X.H.; Dang, Z.B.; Yu, L.H.; Wang, X.B.; Jiang, Y.Y.; Yang, Z.Y. Effects of adjuvant traditional Chinese medicine therapy on long-term survival in patients with hepatocellular carcinoma. Phytomedicine 2019, 62, 152930. [Google Scholar] [CrossRef]
- Liang, N.; Ma, Y.F.; Wang, J.Y.; Li, H.Z.; Wang, X.H.; Jiao, L.W.; Liu, B.; Ma, Y.; Zhao, C.; Luo, X.F.; et al. Traditional Chinese Medicine guidelines for coronavirus disease 2019. J. Tradit. Chin. Med. 2020, 40, 891–896. [Google Scholar]
- Wang, W.Y.; Xie, Y.; Zhou, H.; Liu, L. Contribution of traditional Chinese medicine to the treatment of COVID-19. Phytomedicine 2021, 85, 153279. [Google Scholar] [CrossRef]
- Ni, L.Q.; Chen, L.L.; Huang, X.; Han, C.P.; Xu, J.R.; Zhang, H.; Luan, X.; Zhao, Y.F.; Xu, J.G.; Yuan, W.A.; et al. Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm. Sin. B 2020, 10, 1149–1162. [Google Scholar] [CrossRef]
- Gyamfi, E.T. Metals and metalloids in traditional medicines (Ayurvedic medicines, nutraceuticals and traditional Chinese medicines). Environ. Sci. Pollut. Res. 2019, 26, 15767–15778. [Google Scholar] [CrossRef]
- Wu, X.B.; Xue, J. Heavy Metals Contamination Status and Analyses of Traditional Chinese Medicine. Asian J. Chem. 2013, 25, 19–22. [Google Scholar] [CrossRef]
- Cooper, K.; Noller, B.; Connell, D.; Yu, J.; Sadler, R.; Olszowy, H.; Golding, G.; Tinggi, U.; Moore, M.R.; Myers, S. Public health risks from heavy metals and metalloids present in traditional Chinese medicines. J. Toxicol. Environ. Health-Part A-Curr. Issues 2007, 70, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.T.; Qu, H.R.; Jin, H.Y.; Zhang, L.; Luo, F.Y.; Yu, K.Z.; Gao, F.; Wang, Q.; Sun, L.; He, H.Z.; et al. Innovative health risk assessments of heavy metals based on bioaccessibility due to the consumption of traditional animal medicines. Environ. Sci. Pollut. Res. 2020, 27, 22593–22603. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.Y.; Park, C.; Kung, J.Y.C.; Bou-Chacra, N.A.; Doschak, M.; Lobenberg, R. Traditional Chinese Medicine “Pill”, an Ancient Dosage Form with Surprising Modern Pharmaceutical Characteristics. Pharm. Res. 2021, 38, 199–211. [Google Scholar] [CrossRef]
- Chen, Y.G.; He, X.L.S.; Huang, J.H.; Luo, R.; Ge, H.Z.; Wolowicz, A.N.; Wawrzkiewicz, M.; Gladysz-Plaska, A.; Li, B.; Yu, Q.X.; et al. Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotoxicol. Environ. Saf. 2021, 219, 112336. [Google Scholar] [CrossRef]
- Sheik, A.; Kim, K.; Varaprasad, G.L.; Lee, H.; Kim, S.; Kim, E.; Shin, J.-Y.; Oh, S.Y.; Huh, Y.S. The anti-cancerous activity of adaptogenic herb Astragalus membranaceus. Phytomedicine 2021, 91, 153698. [Google Scholar] [CrossRef]
- Yu, W.; Yang, Y.; Zhou, Q.; Huang, X.; Huang, Z.; Li, T.; Wu, Q.; Zhou, C.; Ma, Z.; Lin, H. Effects of dietary Astragalus polysaccharides on growth, health and resistance to Vibrio harveyi of Lates calcarifer. Int. J. Biol. Macromol. 2022, 207, 850–858. [Google Scholar] [CrossRef]
- Simayi, Z.; Rozi, P.; Yang, X.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.; Ma, S.; Askar, G.; Yadikar, N. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: Systematic review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.; Gao, T.; Chen, Y.; Yang, Q.; Fu, C.; Zhu, Y.; Wang, F.; Liao, W. Isatidis Radix and Isatidis Folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 283, 114648. [Google Scholar] [CrossRef]
- Lin, L.; Dai, F.; Ren, G.Q.; Wei, J.J.; Chen, Z.; Tang, X.Y. Efficacy of lianhuaqingwen granules in the management of chronic rhinosinusitis without nasal polyps. Am. J. Otolaryngol. 2020, 41, 102311. [Google Scholar] [CrossRef]
- Chen, T.B.; Wei, C.Y.; Huang, Z.C.; Huang, Q.F.; Lu, Q.G.; Fan, Z.L. Arsenic hyperaccumulator Pteris vittata L. and its arsenic accumulation. Chin. Sci. Bull. 2002, 47, 902–905. [Google Scholar] [CrossRef]
- Wan, X.M.; Gu, G.Q.; Lei, M.; Zeng, W.B. Bioaccessibility of metals/metalloids in willow catkins collected in urban parks of Beijing and their health risks to human beings. Sci. Total Environ. 2020, 717, 137240. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, L.; Gao, Q.; Wang, J.; Li, X.; Wang, H.; Liu, Y.; Lin, H.; Liu, J.; Wang, X.; et al. A plasma membrane transporter coordinates phosphate reallocation and grain filling in cereals. Nat. Genet. 2021, 53, 906. [Google Scholar] [CrossRef]
- Dong, C.; He, F.; Berkowitz, O.; Liu, J.; Cao, P.; Tang, M.; Shi, H.; Wang, W.; Li, Q.; Shen, Z.; et al. Alternative Splicing Plays a Critical Role in Maintaining Mineral Nutrient Homeostasis in Rice (Oryza sativa). Plant Cell 2018, 30, 2267–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijovilovich, A.; Morina, F.; Bokhari, S.N.; Wolff, T.; Kupper, H. Analysis of trace metal distribution in plants with lab-based microscopic X-ray fluorescence imaging. Plant Methods 2020, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- ISO 18664:2015; Traditional Chinese Medicine—Determination of Heavy Metals in Herbal Medicines Used in Traditional Chinese Medicine; ISO: Geneva, Switzerland, 2015.
- China, National Health Commission of the People’s Republic of China and National Food and Medical Products Administration. Standard. National Food Safety Standards—Quantity of Pollutants in Food (GB 2762-2017). 2017. Available online: https://sppt.cfsa.net.cn:8086/db?type=2&guid=D5921FFE-BD08-4D34-AE26-CF9CA4FEB001 (accessed on 20 November 2022).
- Nan, G.J.; Meng, X.X.; Song, N.; Liu, Z.Z.; Liu, Y.; Yang, G.D. Fractionation analysis and health risk assessment of heavy metals in six traditional Chinese medicines. Environ. Sci. Pollut. Res. 2020, 27, 10308–10316. [Google Scholar] [CrossRef]
- Wen, H.; Wang, H.X.; Yuan, L.M.; Jiang, Y.F. Status and risk evaluation of heavy metals in soil and traditional Chinese medicine in Longxi, Gansu Province, China. In Proceedings of the International Workshop on Green Energy, Environment and Sustainable Development, Weihai, China, 28–30 August 2020. [Google Scholar]
- Li, W.; Chen, G.; Yu, Q.; Yu, R.; Bao, H.; Yu, T. Heavy Metal Contents and Evaluation of Soil and Root of Glycyrrhiza uralensis Fisch, in Different Producing Regions in Inner Mongolia. J. Inn. Mong. Univ. Nat. Sci. Ed. 2014, 45, 661–669. [Google Scholar]
- Han, L.Q.; Dong, S.F.; Liu, J.H. Determination of the contents of trace elements in Chinese herbal medicines for treating respiratory system diseases. Spectrosc. Spectr. Anal. 2008, 28, 453–455. [Google Scholar]
- Harris, E.S.J.; Cao, S.G.; Littlefield, B.A.; Craycroft, J.A.; Scholten, R.; Kaptchuk, T.; Fu, Y.L.; Wang, W.Q.; Liu, Y.; Chen, H.B.A.; et al. Heavy metal and pesticide content in commonly prescribed individual raw Chinese Herbal Medicines. Sci. Total Environ. 2011, 409, 4297–4305. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Zhu, Y.; Yan, F.; Su, Y.; Zhu, Y.; Zhang, Z.; Zuo, C.; Wu, H.; Zhang, Y.; Kan, J.; et al. The difference of origin and extraction method significantly affects the intrinsic quality of licorice: A new method for quality evaluation of homologous materials of medicine and food. Food Chem. 2021, 340, 127907. [Google Scholar] [CrossRef]
- Kan, W.L.T.; Ma, B.; Lin, G. Sulfur fumigation processing of traditional Chinese medicinal herbs: Beneficial or detrimental? Front. Pharmacol. 2011, 2, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, S.-Y.; Zhu, J.-H.; Kong, M.; Zhou, S.-S.; Li, S.-L.; Zhu, H. Effects and contributory factors of sulfur-fumigation on the efficacy and safety of medicinal herbs evaluated by meta-analysis. J. Ethnopharmacol. 2022, 293, 115250. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, Q.; Flower, A.; Lewith, G.; Liu, J.P. Comparison of effectiveness and safety between granules and decoction of Chinese herbal medicine: A systematic review of randomized clinical trials. J. Ethnopharmacol. 2012, 140, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.S.; Bai, M.; Miao, M.S. Current Research Situation of Multipurpose Use of Traditional Chinese Medicine. In Proceedings of the 3rd International Conference on Green Materials and Environmental Engineering (GMEE), Beijing, China, 22 October 2017; pp. 272–276. [Google Scholar]
- Bai, M.; Tian, S.; Miao, M.S.; Jiao, J.J.; Li, Y.; Destech Publicat, I. New Thoughts on Traditional Chinese Medicine Toxicity and the Toxic Traditional Chinese Medicine. In Proceedings of the International Conference on Advanced Education and Management (ICAEM), Guilin, China, 15–16 May 2015; pp. 164–168. [Google Scholar]
- Wang, W.L.; Liang, Z.S.; Tan, Y.; Duan, Q.M. Determination of mineral elements in different part of Astragalus membranaceus (Fisch.) by FAAS. Spectrosc. Spectr. Anal. 2008, 28, 1168–1171. [Google Scholar]
- Cheenkachorn, K.; Paulraj, M.G.; Tantayotai, P.; Phakeenuya, V.; Sriariyanun, M. Characterization of biologically active compounds from different herbs: Influence of drying and extraction methods. J. Indian Chem. Soc. 2022, 99, 100297. [Google Scholar] [CrossRef]






| Element | Pb | As | Cd | Cu |
|---|---|---|---|---|
| China | 5 | 2 | 1 | |
| WHO | 10 | 0.3 | ||
| Import and export * | 5 | 2 | 0.3 | 20 |
| ISO18664 2015 | 10 | 4 | 2 | |
| Taiwan authority | 5 | 3 | 1 | |
| Hongkong | 5 | 2 | 1 | 20 |
| Japan | 20 | 5 | 10 | |
| USA | 5 | 0.5 | ||
| India | 10 | 3 | 0.3 | |
| Canada | 10 | 5 | 0.3 | 2 |
| Astragalus | Glycyrrhizae | Isatis | |
|---|---|---|---|
| Al | 2.47 ± 0.12 | 1.94 ± 0.08 | 1.15 ± 0.08 |
| O | 73.7 ± 1.3 | 70.3 ± 5.8 | 72.2 ± 5.9 |
| Mg | 0.67 ± 0.02 | 1.15 ± 0.10 | 0.55 ± 0.06 |
| Na | 0.22 ± 0.05 | 0.48 ± 0.07 | 0.15 ± 0.04 |
| Si | 8.14 ± 058 | 7.18 ± 1.01 | 3.99 ± 0.19 |
| P | 1.03 ± 0.06 | 0.65 ± 0.08 | 1.26 ± 0.22 |
| S | 0.48 ± 0.04 | 1.10 ± 0.04 | 3.23 ± 0.26 |
| K | 9.46 ± 1.24 | 7.03 ± 1.01 | 9.90 ± 1.05 |
| Ca | 2.42 ± 0.11 | 8.82 ± 1.25 | 6.29 ± 0.99 |
| Mn | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 |
| Fe | 1.08 ± 0.05 | 0.75 ± 0.08 | 0.82 ± 0.10 |
| Cu | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Zn | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.08 ± 0.01 |
| Cl | 0.28 ± 0.02 | 0.55 ± 0.09 | 0.34 ± 0.06 |
| Al | O | Mg | Na | Si | P | S | K | Ca | Mn | Fe | Cu | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | 0.893 * | ||||||||||||
| Mg | 0.841 * | 0.852 * | |||||||||||
| Na | 0.765 | 0.730 | 0.980 ** | ||||||||||
| Si | 0.996 ** | 0.901 * | 0.884 * | 0.817 * | |||||||||
| P | 0.718 | 0.920 ** | 0.584 | 0.410 | 0.705 | ||||||||
| S | 0.185 | 0.606 | 0.334 | 0.192 | 0.203 | 0.764 | |||||||
| K | 0.832 * | 0.976 ** | 0.716 | 0.562 | 0.826 * | 0.981 ** | 0.677 | ||||||
| Ca | 0.551 | 0.761 | 0.884 * | 0.855 * | 0.612 | 0.578 | 0.639 | 0.649 | |||||
| Mn | 0.837 * | 0.991 ** | 0.785 | 0.645 | 0.841 * | 0.961 ** | 0.692 | 0.992 ** | 0.737 | ||||
| Fe | 0.937 ** | 0.970 ** | 0.763 | 0.630 | 0.926 ** | 0.915 * | 0.478 | 0.970 ** | 0.585 | 0.959 ** | |||
| Cu | 0.838 * | 0.833 * | 0.999 ** | 0.986 ** | 0.882 * | 0.553 | 0.296 | 0.691 | 0.872 * | 0.761 | 0.746 | ||
| Zn | 0.415 | 0.765 | 0.424 | 0.254 | 0.416 | 0.918 ** | 0.955 ** | 0.847 * | 0.610 | 0.842 * | 0.693 | 0.387 | |
| Cl | 0.763 | 0.868 * | 0.979 ** | 0.942 ** | 0.811 | 0.645 | 0.506 | 0.749 | 0.957 ** | 0.822 * | 0.745 | 0.971 ** | 0.559 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Zeng, W. Composition of Three Common Chinese Herbal Medicines and the Influence of Preparation Types on the Bioaccessibility of Trace Elements. Toxics 2022, 10, 719. https://doi.org/10.3390/toxics10120719
Wan X, Zeng W. Composition of Three Common Chinese Herbal Medicines and the Influence of Preparation Types on the Bioaccessibility of Trace Elements. Toxics. 2022; 10(12):719. https://doi.org/10.3390/toxics10120719
Chicago/Turabian StyleWan, Xiaoming, and Weibin Zeng. 2022. "Composition of Three Common Chinese Herbal Medicines and the Influence of Preparation Types on the Bioaccessibility of Trace Elements" Toxics 10, no. 12: 719. https://doi.org/10.3390/toxics10120719