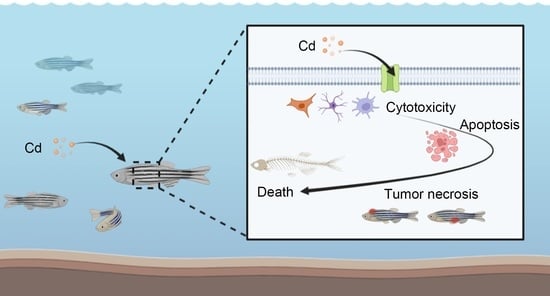
Toxic Effects of Cadmium on Fish
Abstract
:1. Introduction
2. Detection Methods and Accumulation Effects of Cadmium in Fish
2.1. Cadmium Accumulation Induces Cytotoxicity in Fish
2.2. Test Method for Cadmium Accumulation in Fish
2.3. The Accumulation of Cadmium in Fish Was Influenced by Feeding Habits and Routes
3. Toxic Effects of Cadmium Accumulation on Fish
3.1. Cadmium Toxicity Damages Fish Tissue Structure
3.2. Cadmium Toxicity Damages Fish Reproduction, Development and Endocrine System
3.3. Cadmium Toxicity Damages the Immune System of Fish
3.4. Cadmium Toxicity Damages the Energy Metabolism System of Fish
3.5. Cadmium Toxicity Affects Nervous System Development in Fish
3.6. Cadmium Toxicity Leads to Changes in Blood Plasma Parameters
4. Mechanism of the Toxic Effects of Cadmium Exposure on Fish
4.1. Cadmium Toxicity Leads to Oxidative Damage in Fish
4.2. Cadmium Toxicity Affected the Expression of Stress Genes in Fish
4.3. Cadmium Toxicity Inhibits Multiple Enzyme Activities
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cd | cadmium |
| MT | metallothionein |
| AAS | atomic absorption spectroscopy |
| ICP | inductively coupled plasma |
| GFAAS | graphite furnace atomic absorption spectrometry |
| FAAS | flame atomic absorption spectrometry |
| ICP‒MS | inductively coupled plasma‒mass spectrometry |
| ICP‒OES | inductively coupled plasma optical emission spectrometry |
| HPG | hypothalamic–pituitary–gonadal axis |
| GSI | gonad somatic index |
| AChE | acetylcholinesterase |
| CaM | calmodulin |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| MDA | malondialdehyde |
| GR | glutathione reductase |
| SOD | superoxide dismutase |
| CAT | catalase |
| GPx | glutathione peroxidase |
References
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Verma, A.; Jaiswal, P. Detrimental Effects of Heavy Metals in Soil, Plants, and Aquatic Ecosystems and in Humans. J. Environ. Pathol. Toxicol. Oncol. Off. Organ Int. Soc. Environ. Toxicol. Cancer 2018, 37, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic life and Human Health. Int. J. Environ. Res. Public Health 2020, 17, E2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, N.; Peiris-John, R.; Wickremasinghe, R.; Senanayake, H.; Sathiakumar, N. Cadmium a metalloestrogen: Are we convinced? J. Appl. Toxicol. 2012, 32, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Cadmium toxicity and treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef] [PubMed]
- Mezynska, M.; Brzóska, M.M. Environmental exposure to cadmium-a risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. Int. 2018, 25, 3211–3232. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, D.; Shi, X.; Zhao, H.; Liu, Z. Cadmium toxicity: A role in bone cell function and teeth development. Sci. Total Environ. 2021, 769, 144646. [Google Scholar] [CrossRef] [PubMed]
- Matović, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6, E15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on Cadmium Exposure in the Population and Intervention Strategies Against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef]
- Garner, R.; Levallois, P. Cadmium levels and sources of exposure among Canadian adults. Health Rep. 2016, 27, 10–18. [Google Scholar]
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium: Mitigation strategies to reduce dietary exposure. J. Food Sci. 2020, 85, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Mielcarek, K.; Nowakowski, P.; Puścion-Jakubik, A.; Gromkowska-Kępka, K.J.; Soroczyńska, J.; Markiewicz-Żukowska, R.; Naliwajko, S.K.; Grabia, M.; Bielecka, J.; Żmudzińska, A.; et al. Arsenic, cadmium, lead and mercury content and health risk assessment of consuming freshwater fish with elements of chemometric analysis. Food Chem. 2022, 379, 132167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, P.; Wang, Y.; Zhu, Z.; Lao, F.; Liu, X.; Cong, W.; Chen, C.; Gao, Y.; Liu, Y. The influence on cell cycle and cell division by various cadmium-containing quantum dots. Small 2013, 9, 2440–2451. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, H.; Wu, L.; He, L.; Wang, Y.; Ou, Y.; Yang, H.; Peng, S.; Chen, F.; Wang, X.; et al. Cadmium cytotoxicity and possible mechanisms in human trophoblast HTR-8/SVneo cells. Environ. Toxicol. 2021, 36, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.P.; Ferrari, L.; Eissa, B.L. Acute toxicity of cadmium to freshwater fishes and its relationship with body size and respiratory strategy. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2021, 248, 109109. [Google Scholar] [CrossRef] [PubMed]
- Noor, Z.; Khan, S.A.; Noor, M. Assessment of cadmium toxicity and its possible effects on goldfish (Carassius auratus), employing microscopy and biochemical techniques. Microsc. Res. Tech. 2020, 83, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-L.; Peng, L.-B.; Xia, L.-P.; Li, J.; Zhu, Q.-L. Effects of continuous and intermittent cadmium exposure on HPGL axis, GH/IGF axis and circadian rhythm signaling and their consequences on reproduction in female zebrafish: Biomarkers independent of exposure regimes. Chemosphere 2021, 282, 130879. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yin, Y.; Li, Y.; Guan, L.; Zhang, P.; Qin, Y.; Wang, Y.; Li, Y. Cadmium exposure affects growth performance, energy metabolism, and neuropeptide expression in Carassius auratus gibelio. Fish Physiol. Biochem. 2020, 46, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.T.A.; Banaee, M.; Sureda, A. Genotoxicity, oxidative stress, and biochemical biomarkers of exposure to green synthesized cadmium nanoparticles in Oreochromis niloticus (L.). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2021, 242, 108942. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, Z.; Yang, F.; Zhu, M.; Cao, J.; Chen, J.; Lin, Y.; Guo, S.; Li, J.; Liu, Z. Cadmium disturbs epigenetic modification and induces DNA damage in mouse preimplantation embryos. Ecotoxicol. Environ. Saf. 2021, 219, 112306. [Google Scholar] [CrossRef] [PubMed]
- Tilton, S.C.; Foran, C.M.; Benson, W.H. Effects of cadmium on the reproductive axis of Japanese medaka (Oryzias latipes). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2003, 136, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.T.K.; Sin, Y.M.; Wong, M.K. The effects of short-term acute cadmium exposure on blue tilapia, Oreochromis aureus. Environ. Biol. Fishes 1993, 37, 67–74. [Google Scholar] [CrossRef]
- Okorie, O.E.; Bae, J.Y.; Lee, J.-H.; Lee, S.; Park, G.-H.; Mohseni, M.; Bai, S.C. Effects of Different Dietary Cadmium Levels on Growth and Tissue Cadmium Content in Juvenile Parrotfish, Oplegnathus fasciatus. Asian-Australas. J. Anim. Sci. 2014, 27, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, E.K.; Lee, A.N.; Lee, J.-Y.; Shim, I.; Kim, P.; Kim, T.-Y.; Kim, K.-T.; Lee, S. Advantages of omics technology for evaluating cadmium toxicity in zebrafish. Toxicol. Res. 2021, 37, 395–403. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, S.-J.; Bae, M.A.; Kim, J.-R.; Cho, K.-H. Cadmium exposure exacerbates severe hyperlipidemia and fatty liver changes in zebrafish via impairment of high-density lipoproteins functionality. Toxicol. Vitr. 2018, 47, 249–258. [Google Scholar] [CrossRef]
- Monaco, A.; Grimaldi, M.C.; Ferrandino, I. Neuroglial alterations in the zebrafish brain exposed to cadmium chloride. J. Appl. Toxicol. 2016, 36, 1629–1638. [Google Scholar] [CrossRef]
- Senger, M.R.; Rosemberg, D.B.; Rico, E.P.; Arizi, M.d.B.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. In vitro effect of zinc and cadmium on acetylcholinesterase and ectonucleotidase activities in zebrafish (Danio rerio) brain. Toxicol. Vitr. 2006, 20, 954–958. [Google Scholar] [CrossRef]
- Chow, E.S.H.; Hui, M.N.Y.; Lin, C.C.; Cheng, S.H. Cadmium inhibits neurogenesis in zebrafish embryonic brain development. Aquat. Toxicol. 2008, 87, 157–169. [Google Scholar] [CrossRef]
- Tu, H.; Fan, C.; Chen, X.; Liu, J.; Wang, B.; Huang, Z.; Zhang, Y.; Meng, X.; Zou, F. Effects of cadmium, manganese, and lead on locomotor activity and neurexin 2a expression in zebrafish. Environ. Toxicol. Chem. 2017, 36, 2147–2154. [Google Scholar] [CrossRef]
- Chouchene, L.; Pellegrini, E.; Gueguen, M.-M.; Hinfray, N.; Brion, F.; Piccini, B.; Kah, O.; Saïd, K.; Messaoudi, I.; Pakdel, F. Inhibitory effect of cadmium on estrogen signaling in zebrafish brain and protection by zinc. J. Appl. Toxicol. 2016, 36, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Hen Chow, E.S.; Cheng, S.H. Cadmium Affects Muscle Type Development and Axon Growth in Zebrafish Embryonic Somitogenesis. Toxicol. Sci. 2003, 73, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Zhou, X.-Y.; Ma, X.-F.; Liu, J.-X. Mechanisms of cadmium-caused eye hypoplasia and hypopigmentation in zebrafish embryos. Aquat. Toxicol. 2015, 167, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Fraysse, B.; Mons, R.; Garric, J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicol. Environ. Saf. 2006, 63, 253–267. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Collins, J.E.; Sealy, I.M.; Wali, N.; Dooley, C.M.; Digby, Z.; Stemple, D.L.; Murphy, D.N.; Billis, K.; Hourlier, T.; et al. A high-resolution mRNA expression time course of embryonic development in zebrafish. ELife 2017, 6, e30860. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, T.-M.; Zhong, Y. Possible molecular mechanism underlying cadmium-induced circadian rhythms disruption in zebrafish. Biochem. Biophys. Res. Commun. 2016, 481, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Matz, C.J.; Krone, P.H. Cell Death, Stress-Responsive Transgene Activation, and Deficits in the Olfactory System of Larval Zebrafish Following Cadmium Exposure. Environ. Sci. Technol. 2007, 41, 5143–5148. [Google Scholar] [CrossRef]
- Veneman, W.J.; Spaink, H.P.; Brun, N.R.; Bosker, T.; Vijver, M.G. Pathway analysis of systemic transcriptome responses to injected polystyrene particles in zebrafish larvae. Aquat. Toxicol. 2017, 190, 112–120. [Google Scholar] [CrossRef]
- Pan, Y.-X.; Luo, Z.; Zhuo, M.-Q.; Wei, C.-C.; Chen, G.-H.; Song, Y.-F. Oxidative stress and mitochondrial dysfunction mediated Cd-induced hepatic lipid accumulation in zebrafish Danio rerio. Aquat. Toxicol. 2018, 199, 12–20. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Yuan, S.-S.; Wu, C.-W.; Li, W.-Y. Chronic waterborne zinc and cadmium exposures induced different responses towards oxidative stress in the liver of zebrafish. Aquat. Toxicol. 2016, 177, 261–268. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Yuan, S.-S.; Wu, C.-W.; Lv, Z.-M. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio). Aquat. Toxicol. 2016, 180, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-L.; Yuan, S.-S.; Wu, C.-W.; Lv, Z.-M.; Zhu, A.-Y. Circadian time-dependent antioxidant and inflammatory responses to acute cadmium exposure in the brain of zebrafish. Aquat. Toxicol. 2017, 182, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Avallone, B.; Agnisola, C.; Cerciello, R.; Panzuto, R.; Simoniello, P.; Cretì, P.; Motta, C.M. Structural and functional changes in the zebrafish (Danio rerio) skeletal muscle after cadmium exposure. Cell Biol. Toxicol. 2015, 31, 273–283. [Google Scholar] [CrossRef]
- Wu, S.M.; Tsai, P.J.; Chou, M.Y.; Wang, W.-D. Effects of Maternal Cadmium Exposure on Female Reproductive Functions, Gamete Quality, and Offspring Development in Zebrafish (Danio rerio). Arch. Environ. Contam. Toxicol. 2013, 65, 521–536. [Google Scholar] [CrossRef]
- Avallone, B.; Crispino, R.; Cerciello, R.; Simoniello, P.; Panzuto, R.; Maria Motta, C. Cadmium effects on the retina of adult Danio rerio. Comptes Rendus Biol. 2015, 338, 40–47. [Google Scholar] [CrossRef]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem. -Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, W.; Shi, H.; Hou, Y.; Xu, Q. Calcium homeostasis disruption-a bridge connecting cadmium-induced apoptosis, autophagy and tumorigenesis. Oncol. Res. Treat. 2015, 38, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.; Botana, D.; Piñero, S.; Proverbio, F.; Marín, R. Cadmium inhibits motility, activities of plasma membrane Ca2+-ATPase and axonemal dynein-ATPase of human spermatozoa. Andrologia 2016, 48, 464–469. [Google Scholar] [CrossRef]
- Chmielowska-Bąk, J.; Izbiańska, K.; Deckert, J. The toxic Doppelganger: On the ionic and molecular mimicry of cadmium. Acta Biochim. Pol. 2013, 60, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Chao, S.H.; Zysk, J.R.; Cheung, W.Y. Stimulation of calmodulin by cadmium ion. Arch. Toxicol. 1985, 57, 205–211. [Google Scholar] [CrossRef]
- Templeton, D.M.; Liu, Y. Effects of cadmium on the actin cytoskeleton in renal mesangial cells. Can. J. Physiol. Pharmacol. 2013, 91, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Song, X.; Chen, L.; Hu, D.; Hua, L.; Cui, Y.; Liu, J.; An, Z.; Yin, Z.; Ning, H. Cadmium induces actin cytoskeleton alterations and dysfunction in Neuro-2a cells. Environ. Toxicol. 2019, 34, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Adiele, R.C.; Stevens, D.; Kamunde, C. Reciprocal enhancement of uptake and toxicity of cadmium and calcium in rainbow trout (Oncorhynchus mykiss) liver mitochondria. Aquat. Toxicol. 2010, 96, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Nigam, D.; Shukla, G.S.; Agarwal, A.K. Glutathione depletion and oxidative damage in mitochondria following exposure to cadmium in rat liver and kidney. Toxicol. Lett. 1999, 106, 151–157. [Google Scholar] [CrossRef]
- Mao, W.P.; Zhang, N.N.; Zhou, F.Y.; Li, W.X.; Liu, H.Y.; Feng, J.; Zhou, L.; Wei, C.J.; Pan, Y.B.; He, Z.J. Cadmium directly induced mitochondrial dysfunction of human embryonic kidney cells. Hum. Exp. Toxicol. 2011, 30, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Gobe, G.; Crane, D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol. Lett. 2010, 198, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Y.; Kim, M.; Kim, J.-H.; Lee, M.-O.; Chung, J.-H.; Lee, B.-H. Gene expression profiling in human lung fibroblast following cadmium exposure. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qiu, H.; Yan, Y.; Xie, X. Acute Cd Toxicity, Metal Accumulation, and Ion Loss in Southern Catfish (Silurus meridionalis Chen). Toxics 2021, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Renieri, E.A.; Sfakianakis, D.G.; Alegakis, A.A.; Safenkova, I.V.; Buha, A.; Matović, V.; Tzardi, M.; Dzantiev, B.B.; Divanach, P.; Kentouri, M.; et al. Nonlinear responses to waterborne cadmium exposure in zebrafish. An in vivo study. Environ. Res. 2017, 157, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Yusof, S. Effect of mercury and cadmium on early life stages of Java medaka (Oryzias javanicus): A potential tropical test fish. Mar. Pollut. Bull. 2011, 63, 347–349. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Angulo, C.; Sanchez, V.; Cuesta, A.; Cruz, A. Methylmercury, cadmium and arsenic (III)-induced toxicity, oxidative stress and apoptosis in Pacific red snapper leukocytes. Aquat. Toxicol. 2019, 213, 105223. [Google Scholar] [CrossRef]
- Jurgelėnė, Ž.; Stankevičiūtė, M.; Kazlauskienė, N.; Baršienė, J.; Jokšas, K.; Markuckas, A. Toxicological Potential of Cadmium Impact on Rainbow Trout (Oncorhynchus mykiss) in Early Development. Bull. Environ. Contam. Toxicol. 2019, 103, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Shuhaimi-Othman, M.; Yakub, N.; Ramle, N.-A.; Abas, A. Comparative toxicity of eight metals on freshwater fish. Toxicol. Ind. Health 2015, 31, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Robohm, R.A. Paradoxical effects of cadmium exposure on antibacterial antibody responses in two fish species: Inhibition in cunners (Tautogolabrus adspersus) and enhancement in striped bass (Morone saxatilis). Vet. Immunol. Immunopathol. 1986, 12, 251–262. [Google Scholar] [CrossRef]
- Garcia-Santos, S.; Fontaínhas-Fernandes, A.; Wilson, J.M. Cadmium tolerance in the Nile tilapia (Oreochromis niloticus) following acute exposure: Assessment of some ionoregulatory parameters. Environ. Toxicol. 2006, 21, 33–46. [Google Scholar] [CrossRef]
- Delahaut, V.; Rašković, B.; Salvado, M.S.; Bervoets, L.; Blust, R.; De Boeck, G. Toxicity and bioaccumulation of Cadmium, Copper and Zinc in a direct comparison at equitoxic concentrations in common carp (Cyprinus carpio) juveniles. PLoS ONE 2020, 15, e0220485. [Google Scholar] [CrossRef] [Green Version]
- Annune, P.A.; Ebele, S.O.; Oladimeji, A.A. Acute toxicity of cadmium to juveniles of Clarias gariepinus (Teugels) and Oreochromis niloticus (Trewavas). J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1994, 29, 1357–1365. [Google Scholar] [CrossRef]
- Dutta, T.K.; Kaviraj, A. Acute toxicity of cadmium to fish Labeo rohita and copepod Diaptomus forbesi pre-exposed to CaO and KMnO4. Chemosphere 2001, 42, 955–958. [Google Scholar] [CrossRef]
- Roy, S.; Karmakar, D.; Pal, S. Acute Toxicity Bioassay and Determination of LC50 of Cadmium Chloride in Trichogaster (Colisa) fasciata. Appl. Biochem. Biotechnol. 2022, 194, 3890–3900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, H.; Meng, Y.; Jin, G.; Zhu, M. The toxicity of cadmium (Cd2+) towards embryos and pro-larva of soldatov’s catfish (Silurus soldatovi). Ecotoxicol. Environ. Saf. 2012, 80, 258–265. [Google Scholar] [CrossRef]
- Andersen, O. Chelation of cadmium. Environ. Health Perspect. 1984, 54, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Sandbichler, A.M.; Höckner, M. Cadmium Protection Strategies—A Hidden Trade-Off? Int. J. Mol. Sci. 2016, 17, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyrzynska, K.; Kilian, K. On-line sorption-based systems for determination of cadmium with atomic spectrometry detectors. Water Res. 2007, 41, 2839–2851. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; de Andrade, J.B.; Maria das Graças, A.K.; Pereira, M.D.G.; Lemos, V.A.; dos Santos, W.N.L.; Rodrigues, F.D.M.; Souza, A.S.; Ferreira, H.S.; da Silva, E.G.P. Review of procedures involving separation and preconcentration for the determination of cadmium using spectrometric techniques. J. Hazard. Mater. 2007, 145, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.A.; de Carvalho, A.L. Determination of cadmium and lead in human biological samples by spectrometric techniques: A review. Environ. Monit. Assess. 2010, 171, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yuan, Z.; Xiaona, H.; Wei, M. Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotoxicol. Environ. Saf. 2012, 81, 55–64. [Google Scholar] [CrossRef]
- Weber, P.; Behr, E.R.; Knorr, C.D.L.; Vendruscolo, D.S.; Flores, E.M.M.; Dressler, V.L.; Baldisserotto, B. Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchem. J. 2013, 106, 61–66. [Google Scholar] [CrossRef]
- Rejomon, G.; Nair, M.; Joseph, T. Trace metal dynamics in fishes from the southwest coast of India. Environ. Monit. Assess. 2010, 167, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Ayyat, M.S.; Mahmoud, H.K.; El-Hais, A.E.-A.M.; Abd El-Latif, K.M. The role of some feed additives in fish fed on diets contaminated with cadmium. Environ. Sci. Pollut. Res. Int. 2017, 24, 23636–23645. [Google Scholar] [CrossRef]
- Junejo, S.H.; Baig, J.A.; Kazi, T.G.; Afridi, H.I. Cadmium and Lead Hazardous Impact Assessment of Pond Fish Species. Biol. Trace Elem. Res. 2019, 191, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Klinck, J.S.; Green, W.W.; Mirza, R.S.; Nadella, S.R.; Chowdhury, M.J.; Wood, C.M.; Pyle, G.G. Branchial cadmium and copper binding and intestinal cadmium uptake in wild yellow perch (Perca flavescens) from clean and metal-contaminated lakes. Aquat. Toxicol. 2007, 84, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Glover, C.N.; Nicol, J.A.; Wood, C.M. Calcium/cadmium interactions at uptake surfaces in rainbow trout: Waterborne versus dietary routes of exposure. Environ. Toxicol. Chem. 2005, 24, 2954–2964. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, Y.; Xie, X. Tissue-Specific Antioxidative Responses and Cadmium Accumulation in Silurus meridionalis Under Chronic Waterborne Cadmium Exposure. Bull. Environ. Contam. Toxicol. 2018, 100, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mendivil, D.D.; Garcia-Flores, E.; Temores-Pena, J.; Wakida, F.T. Health Risk Assessment of Some Heavy Metals from Canned Tuna and Fish in Tijuana, Mexico. Health Scope 2019, 8, e78956. [Google Scholar] [CrossRef] [Green Version]
- Olson, K.R. Vasculature of the fish gill: Anatomical correlates of physiological functions. J. Electron. Microsc. Tech. 1991, 19, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.; Giari, L.; Depasquale, J.A.; Dezfuli, B.S. European Sea bass gill pathology after exposure to cadmium and terbuthylazine: Expert versus fractal analysis. J. Microsc. 2016, 261, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.; Giari, L.; De Pasquale, J.A.; Sayyaf Dezfuli, B. Local connected fractal dimension analysis in gill of fish experimentally exposed to toxicants. Aquat. Toxicol. 2016, 175, 12–19. [Google Scholar] [CrossRef]
- Garcia-Santos, S.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Varela, J.L.; Mancera, J.M.; Fontaínhas-Fernandes, A.; Wilson, J.M. Metabolic and osmoregulatory changes and cell proliferation in gilthead sea bream (Sparus aurata) exposed to cadmium. Ecotoxicol. Environ. Saf. 2011, 74, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.O.F.; Martinez, C.B.R. Acute effects of cadmium on osmoregulation of the freshwater teleost Prochilodus lineatus: Enzymes activity and plasma ions. Aquat. Toxicol. 2014, 156, 161–168. [Google Scholar] [CrossRef]
- Thophon, S.; Kruatrachue, M.; Upatham, E.S.; Pokethitiyook, P.; Sahaphong, S.; Jaritkhuan, S. Histopathological alterations of white seabass, Lates calcarifer, in acute and subchronic cadmium exposure. Environ. Pollut. 2003, 121, 307–320. [Google Scholar] [CrossRef]
- Yeşilbudak, B.; Erdem, C. Cadmium accumulation in gill, liver, kidney and muscle tissues of common carp, Cyprinus carpio, and Nile tilapia, Oreochromis niloticus. Bull. Environ. Contam. Toxicol. 2014, 92, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Franco-Fuentes, E.; Moity, N.; Ramírez-González, J.; Andrade-Vera, S.; González-Weller, D.; Hardisson, A.; Paz, S.; Rubio, C.; Gutiérrez, A.J. Metal and metalloids concentration in Galapagos fish liver and gonad tissues. Mar. Pollut. Bull. 2021, 173, 112953. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Cheng, J.; Bao, L.; Zhu, X.; Li, H.; Chen, X.; Zhang, Y.; Zhang, J.; Chu, W.; Pan, Y.; et al. Exposure to waterborne cadmium induce oxidative stress, autophagy and mitochondrial dysfunction in the liver of Procypris merus. Ecotoxicol. Environ. Saf. 2020, 204, 111051. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, X. Inconsistent responses of liver mitochondria metabolism and standard metabolism in Silurus meridionalis when exposed to waterborne cadmium. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2018, 214, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, G.; Ishaq, S.; Arshad, M.; Fatima, S.; Kanwal, Z.; Ali, F. Investigation on Immune-Related Protein (Heat Shock Proteins and Metallothionein) Gene Expression Changes and Liver Histopathology in Cadmium-Stressed Fish. BioMed Res. Int. 2022, 2022, 2075791. [Google Scholar] [CrossRef]
- Pinto, G.L.; da Silva Castro, J.; Val, A.L. Copper and cadmium impair sperm performance, fertilization and hatching of oocytes from Amazonian fish Colossoma macropomum. Chemosphere 2021, 266, 128957. [Google Scholar] [CrossRef]
- Sierra-Marquez, L.; Espinosa-Araujo, J.; Atencio-Garcia, V.; Olivero-Verbel, J. Effects of cadmium exposure on sperm and larvae of the neotropical fish Prochilodus magdalenae. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2019, 225, 108577. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, Y.; Wang, S.; Xing, H.; Dong, W.-F. Effect of combined exposure to silica nanoparticles and cadmium chloride on female zebrafish ovaries. Environ. Toxicol. Pharmacol. 2021, 87, 103720. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shan, D.; Zhong, H.; Zhou, Y.; Chen, W.; Cao, J.; Guo, Z.; Xiao, J.; He, F.; He, Y.; et al. Subchronic effects of cadmium on the gonads, expressions of steroid hormones and sex-related genes in tilapia Oreochromis niloticus. Ecotoxicology 2015, 24, 2213–2223. [Google Scholar] [CrossRef]
- Tian, J.; Hu, J.; He, W.; Zhou, L.; Huang, Y. Parental exposure to cadmium chloride causes developmental toxicity and thyroid endocrine disruption in zebrafish offspring. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2020, 234, 108782. [Google Scholar] [CrossRef]
- Guével, R.L.; Petit, F.G.; Goff, P.L.; Métivier, R.; Valotaire, Y.; Pakdel, F. Inhibition of rainbow trout (Oncorhynchus mykiss) estrogen receptor activity by cadmium. Biol. Reprod. 2000, 63, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amutha, C.; Subramanian, P. Cadmium alters the reproductive endocrine disruption and enhancement of growth in the early and adult stages of Oreochromis mossambicus. Fish Physiol. Biochem. 2013, 39, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Nesatyy, V.J.; Ammann, A.A.; Rutishauser, B.V.; Suter, M.J.F. Effect of cadmium on the interaction of 17beta-estradiol with the rainbow trout estrogen receptor. Environ. Sci. Technol. 2006, 40, 1358–1363. [Google Scholar] [CrossRef]
- Sellin, M.K.; Kolok, A.S. Cd exposures in fathead minnows: Effects on adult spawning success and reproductive physiology. Arch. Environ. Contam. Toxicol. 2006, 51, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergilio, C.S.; Moreira, R.V.; Carvalho, C.E.V.; Melo, E.J.T. Evolution of cadmium effects in the testis and sperm of the tropical fish Gymnotus carapo. Tissue Cell 2015, 47, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, C.; Mazumder, R.; Biswas, R.; Sengupta, M. Cadmium exposure induces inflammation through the canonical NF-κΒ pathway in monocytes/macrophages of Channa punctatus Bloch. Fish Shellfish. Immunol. 2021, 110, 116–126. [Google Scholar] [CrossRef]
- Jiaxin, S.; Shengchen, W.; Yirong, C.; Shuting, W.; Shu, L. Cadmium exposure induces apoptosis, inflammation and immunosuppression through CYPs activation and antioxidant dysfunction in common carp neutrophils. Fish Shellfish. Immunol. 2020, 99, 284–290. [Google Scholar] [CrossRef]
- Naz, S.; Hussain, R.; Ullah, Q.; Chatha, A.M.M.; Shaheen, A.; Khan, R.U. Toxic effect of some heavy metals on hematology and histopathology of major carp (Catla catla). Environ. Sci. Pollut. Res. Int. 2021, 28, 6533–6539. [Google Scholar] [CrossRef]
- Gill, T.S.; Pant, J.C. Erythrocytic and leukocytic responses to cadmium poisoning in a freshwater fish, Puntius conchonius ham. Environ. Res. 1985, 36, 327–337. [Google Scholar] [CrossRef]
- Albergoni, V.; Viola, A. Effects of cadmium on lymphocyte proliferation and macrophage activation in catfish, Ictalurus melas. Fish Shellfish. Immunol. 1995, 5, 301–311. [Google Scholar] [CrossRef]
- Tan, X.-Y.; Luo, Z.; Zhang, G.-Y.; Liu, X.-J.; Jiang, M. Effect of dietary cadmium level on the growth, body composition and several hepatic enzymatic activities of juvenile yellow catfish, Pelteobagrus fulvidraco. Aquac. Res. 2010, 41, 1022–1029. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Li, H.; Li, C.; Huo, X.-J.; Hou, L.-P.; Gong, Z. Immune response induced by major environmental pollutants through altering neutrophils in zebrafish larvae. Aquat. Toxicol. 2018, 201, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xia, Z.; Wang, F. Zebrafish in the sea of mineral (iron, zinc, and copper) metabolism. Front. Pharmacol. 2014, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Paul, J.S.; Small, B.C. Chronic exposure to environmental cadmium affects growth and survival, cellular stress, and glucose metabolism in juvenile channel catfish (Ictalurus punctatus). Aquat. Toxicol. 2021, 230, 105705. [Google Scholar] [CrossRef]
- Altarelli, M.; Ben-Hamouda, N.; Schneider, A.; Berger, M.M. Copper Deficiency: Causes, Manifestations, and Treatment. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2019, 34, 504–513. [Google Scholar] [CrossRef]
- Bala, S.; Failla, M.L.; Lunney, J.K. Alterations in splenic lymphoid cell subsets and activation antigens in copper-deficient rats. J. Nutr. 1991, 121, 745–753. [Google Scholar] [CrossRef]
- Livingstone, C. Zinc: Physiology, deficiency, and parenteral nutrition. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2015, 30, 371–382. [Google Scholar] [CrossRef]
- Speakman, J.R. Body size, energy metabolism and lifespan. J. Exp. Biol. 2005, 208, 1717–1730. [Google Scholar] [CrossRef] [Green Version]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Verbost, P.M.; Flik, G.; Lock, R.A.; Wendelaar Bonga, S.E. Cadmium inhibits plasma membrane calcium transport. J. Membr. Biol. 1988, 102, 97–104. [Google Scholar] [CrossRef]
- Peles, J.D.; Pistole, D.H.; Moffe, M. Influence of cadmium concentration and length of exposure on metabolic rate and gill Na+/K+ ATPase activity of golden shiners (Notemigonus crysoleucas). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 156, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M.; Evans, S.; Hughes, F.M. Cadmium-induced apoptosis in oyster hemocytes involves disturbance of cellular energy balance but no mitochondrial permeability transition. J. Exp. Biol. 2004, 207, 3369–3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurochkin, I.O.; Etzkorn, M.; Buchwalter, D.; Leamy, L.; Sokolova, I.M. Top-down control analysis of the cadmium effects on molluscan mitochondria and the mechanisms of cadmium-induced mitochondrial dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R21–R31. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Liu, X.; Zhang, W.; Cao, J.; Li, W.; Li, C.; Lin, Z. Identification of a regulation network in response to cadmium toxicity using blood clam Tegillarca granosa as model. Sci. Rep. 2016, 6, 35704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, A.J.; Planchart, A. The neurological toxicity of heavy metals: A fish perspective. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2018, 208, 12–19. [Google Scholar] [CrossRef]
- Lee, D.-C.; Choi, Y.J.; Kim, J.-H. Toxic effects of waterborne cadmium exposure on hematological parameters, oxidative stress, neurotoxicity, and heat shock protein 70 in juvenile olive flounder, Paralichthysolivaceus. Fish Shellfish. Immunol. 2022, 122, 476–483. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, H.; Wang, Z.; Gao, H.; Liu, J.; Li, K.; Song, Z.; Yuan, C.; Lan, X.; Pan, C.; et al. Developmental exposure to environmental levels of cadmium induces neurotoxicity and activates microglia in zebrafish larvae: From the perspectives of neurobehavior and neuroimaging. Chemosphere 2022, 291, 132802. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hu, J.; Liu, D.; Yin, J.; Chen, M.; Zhou, L.; Yin, H. Cadmium chloride-induced transgenerational neurotoxicity in zebrafish development. Environ. Toxicol. Pharmacol. 2021, 81, 103545. [Google Scholar] [CrossRef]
- Faucher, K.; Fichet, D.; Miramand, P.; Lagardère, J.P. Impact of acute cadmium exposure on the trunk lateral line neuromasts and consequences on the “C-start” response behaviour of the sea bass (Dicentrarchus labrax L.; Teleostei, Moronidae). Aquat. Toxicol. 2006, 76, 278–294. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.L.; Obih, P.O.; Jaiswal, R.; Hartley, W.R.; Thiyagarajah, A. Evaluation of liver and brain esterases in the spotted gar fish (Lepisosteus oculatus) as biomarkers of effect in the lower Mississippi River Basin. Bull. Environ. Contam. Toxicol. 1997, 58, 688–695. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, M.; Pan, H.; Li, S.; Ren, B.; Ren, Z.; Xing, N.; Qi, L.; Ren, Q.; Xu, S.; et al. Does time difference of the acetylcholinesterase (AChE) inhibition in different tissues exist? A case study of zebra fish (Danio rerio) exposed to cadmium chloride and deltamethrin. Chemosphere 2017, 168, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, K.; Wang, R.; Zhang, Y.; Zhou, B. Exposure to cadmium causes inhibition of otolith development and behavioral impairment in zebrafish larvae. Aquat. Toxicol. 2019, 214, 105236. [Google Scholar] [CrossRef] [PubMed]
- Volz, S.N.; Hausen, J.; Nachev, M.; Ottermanns, R.; Schiwy, S.; Hollert, H. Short exposure to cadmium disrupts the olfactory system of zebrafish (Danio rerio)-Relating altered gene expression in the olfactory organ to behavioral deficits. Aquat. Toxicol. 2020, 226, 105555. [Google Scholar] [CrossRef]
- Nogami, E.M.; Kimura, C.C.; Rodrigues, C.; Malagutti, A.R.; Lenzi, E.; Nozaki, J. Effects of dietary cadmium and its bioconcentration in tilapia Oreochromis niloticus. Ecotoxicol. Environ. Saf. 2000, 45, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Kondera, E.; Ługowska, K.; Sarnowski, P. High affinity of cadmium and copper to head kidney of common carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2014, 40, 9–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sövényi, J.; Szakolczai, J. Studies on the toxic and immunosuppressive effects of cadmium on the common carp. Acta Vet. Hung. 1993, 41, 415–426. [Google Scholar]
- Zachoval, R. Clinical meaning of elevated aminotransferases. MMW Fortschr. Der Med. 2021, 163, 51. [Google Scholar] [CrossRef]
- Xing, R.Y.; Whitman, W.B. Characterization of amino acid aminotransferases of Methanococcus aeolicus. J. Bacteriol. 1992, 174, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Vaglio, A.; Landriscina, C. Changes in liver enzyme activity in the teleost Sparus aurata in response to cadmium intoxication. Ecotoxicol. Environ. Saf. 1999, 43, 111–116. [Google Scholar] [CrossRef]
- Zhang, Y. Cell toxicity mechanism and biomarker. Clin. Transl. Med. 2018, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souid, G.; Souayed, N.; Yaktiti, F.; Maaroufi, K. Effect of acute cadmium exposure on metal accumulation and oxidative stress biomarkers of Sparus aurata. Ecotoxicol. Environ. Saf. 2013, 89, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Li, J.; Liu, Y.; Gu, X.; Teng, X. Cadmium-induced Oxidative Stress and Immunosuppression Mediated Mitochondrial Apoptosis via JNK-FoxO3a-PUMA pathway in Common Carp (Cyprinus carpio L.) Gills. Aquat. Toxicol. 2021, 233, 105775. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhu, Q.-L.; Zheng, J.-L.; Wen, Z.-Y. Cadmium induced oxidative stress, endoplasmic reticulum (ER) stress and apoptosis with compensative responses towards the up-regulation of ribosome, protein processing in the ER, and protein export pathways in the liver of zebrafish. Aquat. Toxicol. 2022, 242, 106023. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Wu, Z.; Zheng, R.; Liu, G.; Liu, R.; Wu, S.; Sun, C. Calcium protects bacteria against cadmium stress via reducing nitric oxide production and increasing iron acquisition. Environ. Microbiol. 2021, 23, 3541–3553. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, J.; Zhang, K.; Liu, X.; Li, J. Effects of chronic cadmium poisoning on Zn, Cu, Fe, Ca, and metallothionein in liver and kidney of rats. Biol. Trace Elem. Res. 2012, 149, 57–63. [Google Scholar] [CrossRef]
- Moulis, J.-M. Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2010, 23, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Ma, H.-L.; Deng, Y.-Q.; Feng, J.; Jie, Y.-K.; Guo, Z.-X. Oxidative stress, cell cycle arrest, DNA damage and apoptosis in the mud crab (Scylla paramamosain) induced by cadmium exposure. Chemosphere 2021, 263, 128277. [Google Scholar] [CrossRef]
- Petitjean, Q.; Jean, S.; Gandar, A.; Côte, J.; Laffaille, P.; Jacquin, L. Stress responses in fish: From molecular to evolutionary processes. Sci. Total Environ. 2019, 684, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Sheader, D.L.; Williams, T.D.; Lyons, B.P.; Chipman, J.K. Oxidative stress response of European flounder (Platichthys flesus) to cadmium determined by a custom cDNA microarray. Mar. Environ. Res. 2006, 62, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Rhee, J.-S.; Lee, J.-S.; Dahms, H.-U.; Lee, J.; Han, K.-N.; Lee, J.-S. Effect of cadmium exposure on expression of antioxidant gene transcripts in the river pufferfish, Takifugu obscurus (Tetraodontiformes). Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2010, 152, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Guan, X.; Yao, L.; Zhang, H.; Jin, X.; Han, Y. Effects of Single and Joint Subacute Exposure of Copper and Cadmium on Heat Shock Proteins in Common Carp (Cyprinus carpio). Biol. Trace Elem. Res. 2016, 169, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Sen, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Immunotoxicological effects of cadmium on Labeo rohita, with emphasis on the expression of HSP genes. Fish Shellfish. Immunol. 2016, 54, 164–171. [Google Scholar] [CrossRef]
- Gökalp, F.D.; Doğanlar, O.; Doğanlar, Z.B.; Güner, U. The genotoxic effects of mixture of aluminum, arsenic, cadmium, cobalt, and chromium on the gill tissue of adult zebrafish (Danio rerio, Hamilton 1822). Drug Chem. Toxicol. 2022, 45, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, Y.; Ming, M.; Song, J.; Chen, Z.; Xiao, Z. Amelioration of Cd-induced bioaccumulation, hematological parameters, and heat shock protein-related genes by Vitamin C on common carp. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2022, 258, 109362. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, T.; Cocchieri, R.A.; Fasano, E.; Lucisano, A.; Tafuri, S.; Ferrante, M.C.; Carpenè, E.; Andreani, G.; Isani, G. Cadmium Accumulation and Antioxidant Responses in Sparus aurata Exposed to Waterborne Cadmium. Arch. Environ. Contam. Toxicol. 2012, 62, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.S.; Ribas, J.L.C.; Vicari, T.; Silva, S.B.; Stival, J.; Baldan, A.P.; Domingos, F.X.V.; Grassi, M.T.; Cestari, M.M.; Assis, H.C.S.d. Effects of ecologically relevant concentrations of cadmium in a freshwater fish. Ecotoxicol. Environ. Saf. 2016, 130, 29–36. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Shih, C.-M.; Huang, C.-J.; Lin, C.-M.; Chou, C.-M.; Tsai, M.-L.; Liu, T.P.; Chiu, J.-F.; Chen, C.-T. Effects of cadmium on structure and enzymatic activity of Cu, Zn-SOD and oxidative status in neural cells. J. Cell. Biochem. 2006, 98, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Jihen, E.H.; Imed, M.; Fatima, H.; Abdelhamid, K. Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver of the rat: Effects on the oxidative stress. Ecotoxicol. Environ. Saf. 2009, 72, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, H. Metallothionein, antioxidant enzymes and DNA strand breaks as biomarkers of Cd exposure in a marine crab, Charybdis japonica. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2006, 144, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Dabas, A.; Nagpure, N.S.; Kumar, R.; Kushwaha, B.; Kumar, P.; Lakra, W.S. Assessment of tissue-specific effect of cadmium on antioxidant defense system and lipid peroxidation in freshwater murrel, Channa punctatus. Fish Physiol. Biochem. 2012, 38, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hong, F.; Liu, C.; Wang, Y.; Wu, K.; Gao, F.; Yang, F. Regulative mechanism of Ce3+ relieves DNA damage caused by Cd2+ in the kidney of silver crucian carp. Biol. Trace Elem. Res. 2006, 113, 231–245. [Google Scholar] [CrossRef]



| Control | 1 ppb Cd | 5 ppb Cd | 10 ppb Cd | ||
|---|---|---|---|---|---|
| Reproductive endpoints | Total eggs | 99.2 ± 32.7 | 124.0 ± 29.5 | 134.7 ± 29.5 | 113.0 ± 26.1 |
| Total eggs/day | 8.0 ± 2.0 | 9.2 ± 2.0 | 10.3 ± 1.4 | 8.6 ± 2.0 | |
| Fertilized eggs/day | 7.4 ± 1.8 | 8.9 ± 2.0 | 10.1 ± 1.3 | 8.3 ± 2.2 | |
| Hatched eggs/day | 6.2 ± 1.0 | 7.3 ± 0.9 | 9.4 ± 0.9* | 7.6 ± 2.7 | |
| Days to first hatch | 12.4 ± 2.5 | 11.6 ± 1.5 | 10.5 ± 1.9 | 11.8 ± 2.3 | |
| Percent survival | 0.92 ± 0.4 | 0.88 ± 0.19 | 0.93 ± 0.04 | 0.98 ± 0.02 | |
| Spawning frequency | 12.2 ± 1.5 | 13.4 ± 0.5 | 13.0 ± 1.4 | 13.2 ± 0.7 | |
| Egg size (μm) | 1.34 ± 0.02 | 1.35 ± 0.02 | 1.34 ± 0.03 | 1.36 ± 0.02 | |
| Physiological endpoints (females) | Plasma VTG (IOD/positve) | 0.35 ± 0.19 | 0.40 ± 0.20 | 0.38 ± 0.10 | 0.40 ± 0.15 |
| Hepatic ER (IOD/positive) | 0.79 ± 0.10 | 0.72 ± 0.07 | 0.74 ± 0.06 | 0.74 ± 0.07 | |
| GSI (mm2/mg) | 0.050 ± 0.005 | 0.052 ± 0.008 | 0.051 ± 0.011 | 0.049 ± 0.003 | |
| Plasma E2 (pg/μL) | 29.2 ± 13.9 | 36.6 ± 4.5 | 54.3 ± 7.1 * | 12.4 ± 5.9 * | |
| Plasma T (pg/μL) | 10.3 ± 6.8 | 6.5 ± 2.8 | 15.9 ± 3.6 | 18.4 ± 25.1 | |
| Gonadal E2 (pg) | 314 ± 90 | 58 ± 63 * | 56 ± 50 * | 164 ± 108 * | |
| Gonadal T (pg) | 561 ± 106 | 261 ± 146 * | 167 ± 168 * | 121 ± 62 * | |
| Physiological endpoints (males) | Plasma VTG (IOD/positve) | 0.18 ± 0.02 | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.19 ± 0.01 |
| Hepatic ER (IOD/positive) | 0.78 ± 0.15 | 0.73 ± 0.05 | 0.76 ± 0.05 | 0.76 ± 0.08 | |
| GSI (mm2/mg) | 0.013 ± 0.002 | 0.011 ± 0.002 | 0.007 ± 0.003 * | 0.010 ± 0.003 | |
| Plasma E2 (pg/μL) | 39.1 ± 15.3 | 62.0 ± 17.1 | 65.6 ± 4.4 | 33.0 ± 10.4 | |
| Plasma T (pg/μL) | 7.4 ± 4.7 | 11.9 ± 2.0 | 9.7 ± 3.4 | 28.4 ± 36.8 | |
| Gonadal E2 (pg) | 638 ± 219 | BD * | BD * | 89 ± 154 * | |
| Gonadal T (pg) | 326 ± 109 | 168 ± 98 * | 88 ± 57 * | 115 ± 57 * |
| Toxicity | Ref. | |
|---|---|---|
| Embryos | ||
| Liver | Hepatic lipid accumulation | [26] |
| Nerve | Neuroglia alterations | [27] |
| Increased ATPase activity in brain | [28] | |
| Reduction of neuronal differentiation and axonogenesis | [29] | |
| Interference of neural development | [30] | |
| Anti-estrogen in brain | [31] | |
| Abnormal somite patterning | [32] | |
| Myoskeletal retina | Eye hypoplasia and hypopigmentation | [33] |
| Cardiovascular organ | Heart edema and increased pericardial area | [34] |
| Activation of cell death pathway in olfactory epithelium | [35] | |
| Olfactory organ | Delay in hatching time | [34] |
| Others | Tail and axis malformation | [34] |
| Larvae | ||
| Nerve | Circadian rhythms disruption | [36] |
| Others | Cell death and structural alterations in olfactory epithelium | [37] |
| Adults | ||
| Liver | Carcinogenesis | [38] |
| Hepatic lipid accumulation | [39] | |
| Oxidative damage | [40,41] | |
| Nerve | Oxidative damage | [41,42] |
| Myoskeletal | Structural disorganization, disassembly of muscular myofibrils | [43] |
| Reproductive organ | Pair spawning reduction and teratogenicity | [44] |
| Ovary: oxidative damage | [41] | |
| Retina | Nerve fiber thickening and vacuolating | [45] |
| Species | Concentration | Time | Effect | Ref. |
|---|---|---|---|---|
| Silurus meridionalis | 6.85 mg/L | 96 h | Median lethal | [58] |
| Danio rerio | 25 μg/L | 9 d | Median lethal | [59] |
| Oryzias javanicus | 1.0 ppm | - | Embryo developmental arrest | [60] |
| Lutjanus peru | 0.05 mM | 2 h | Cell viability reduction | [61] |
| Oncorhynchus mykiss | 8 µg /L | 96 h | Embryonic mortality rate (97.5%) | [62] |
| Rasbora sumatrana | 0.1 mg/L | 96 h | Median lethal | [63] |
| Poeciliareticulata | 0.17 mg/L | 96 h | Median lethal | [63] |
| Tautogolabrus adspersus | 26 μg/mL | 96 h | Median lethal | [64] |
| Morone saxatilis | 20 μg/mL | 96 h | Median lethal | [64] |
| Oreochromis niloticus | 14.8 mg/L | 96 h | Median lethal | [65] |
| Cyprinus carpio | 0.20 ± 0.16 μM | 96 h | Median lethal | [66] |
| Clarias gariepinus | 10.85 mg/L | 96 h | Median lethal | [67] |
| Labeo rohita | 89.5 mg/L | 96 h | Median lethal | [68] |
| Trichogaster (Colisa) fasciata | 49.5 mg/L | 96 h | Median lethal | [69] |
| Silurus soldatovi | 2.74 mg/L | 96 h | Median lethal | [70] |
| Species | Cd | ||
|---|---|---|---|
| Min | Max | Mean ± SD | |
| Mako shark (Isurusoxyrinchus) | 0.001 | 0.003 | 0.0023 ± 0.0005 |
| Yellowfin tuna (Thunnusalbacares) | 0.001 | 0.002 | 0.0019 ± 0.0001 |
| Soupfin shark (Galeorhinusgaleus) | 0.001 | 0.002 | 0.0018 ± 0.0002 |
| Swordfish (Xiphias gladius) | 0.001 | 0.003 | 0.0022 ± 0.0004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Q.; Li, Y.; Bi, L.; Jin, L.; Peng, R. Toxic Effects of Cadmium on Fish. Toxics 2022, 10, 622. https://doi.org/10.3390/toxics10100622
Liu Y, Chen Q, Li Y, Bi L, Jin L, Peng R. Toxic Effects of Cadmium on Fish. Toxics. 2022; 10(10):622. https://doi.org/10.3390/toxics10100622
Chicago/Turabian StyleLiu, Yinai, Qianqian Chen, Yaoqi Li, Liuliu Bi, Libo Jin, and Renyi Peng. 2022. "Toxic Effects of Cadmium on Fish" Toxics 10, no. 10: 622. https://doi.org/10.3390/toxics10100622