Polyphenols of Traditional Apple Varieties in Interaction with Barley β-Glucan: A Study of the Adsorption Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apples and Polyphenol Extraction
2.3. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) Method
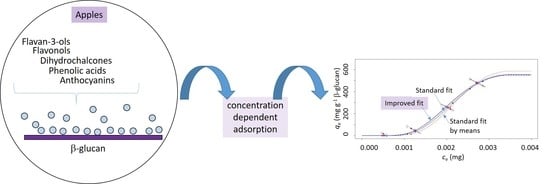
2.4. Adsorption
2.5. Adsorption Isotherms
2.6. Statistical Analysis
3. Results
3.1. Polyphenols and Their Adsorption Capacity
3.2. Adsorption Isotherms
3.2.1. Langmuir Adsorption Isotherm
3.2.2. Dubinin–Radushkevich Adsorption Isotherm
3.2.3. Hill Adsorption Isotherm
3.2.4. Comparison of Adsorption Isotherm Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Polyphenols as food bioactive compounds in the context of Autism Spectrum Disorders: A critical mini-review. Neurosci. Biobehav. Rev. 2019, 102, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Wu, X.; Li, M.; Xiao, Z.; Daglia, M.; Dragan, S.; Delmas, D.; Vong, C.T.; Wang, Y.; Zhao, Y.; Shen, J.; et al. Dietary polyphenols for managing cancers: What have we ignored? Trends Food. Sci. Technol. 2020, 101, 150–164. [Google Scholar] [CrossRef]
- Matacchione, G.; Gurău, F.; Baldoni, S.; Prattichizzo, F.; Silvestrini, A.; Giuliani, A.; Pugnaloni, A.; Espinosa, E.; Amenta, F.; Bonafe, M.; et al. Pleiotropic effects of polyphenols on glucose and lipid metabolism: Focus on clinical trials. Ageing Res. Rev. 2020, 61, 101074. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Zhao, J.; Wang, H.; Chen, F.; Meng, H.; Chen, L.; Zhang, Q.; Yan, J.; Yuan, L. Apple polyphenol relieves hypoxia-induced pulmonary arterial hypertension via pulmonary endothelium protection and smooth muscle relaxation: In vivo and in vitro studies. Biomed. Pharmacother. 2018, 107, 937–944. [Google Scholar] [CrossRef]
- Tamura, Y.; Tomiya, S.; Takegaki, J.; Kouzaki, K.; Tsutaki, A.; Nakazato, K. Apple polyphenols induce browning of white adipose tissue. J. Nutr. Biochem. 2020, 77, 108299. [Google Scholar] [CrossRef]
- Kschonsek, J.; Wiegand, C.; Hipler, U.C.; Böhm, V. Influence of polyphenolic content on the in vitro allergenicity of old and new apple cultivars: A pilot study. Nutrition 2019, 58, 30–35. [Google Scholar] [CrossRef]
- Vegro, M.; Eccher, G.; Populin, F.; Sorgato, C.; Savazzini, F.; Pagliarani, G.; Tartarini, S.; Pasini, G.; Curioni, A.; Antico, A.; et al. Old Apple (Malus domestica L. Borkh) varieties with hypoallergenic properties: An integrated approach for studying apple allergenicity. J. Agric. Food Chem. 2016, 64, 9224–9236. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [Green Version]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanism and consequences in food processing and digestion. Trends Food. Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Gao, R.; Liu, H.; Peng, Z.; Wu, Z.; Wang, Y.; Zhao, G. Adsorption of (-)-epigallocatechin-3-gallate (EGCG) onto oat β-glucan. Food Chem. 2012, 132, 1936–1943. [Google Scholar] [CrossRef]
- Liu, C.; Ge, S.; Yang, J.; Xu, Y.; Zhao, M.; Xiong, L.; Sun, Q. Adsorption mechanism of polyphenols onto starch nanoparticles and enhanced antioxidant activity under adverse conditions. J. Funct. Food 2016, 26, 632–644. [Google Scholar] [CrossRef]
- Liu, D.; Martinez-Sanz, M.; Lopez-Sanchez, P.; Gilbert, E.P.; Gidley, M.J. Adsorption behavior of polyphenols on cellulose is affected by processing history. Food Hydrocoll. 2017, 63, 496–507. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef]
- Phan, A.D.T.; D’Arcy, B.R.; Gidley, M.J. Polyphenol-cellulose interactions: Effects of pH, temperature and salt. Int. J. Food Sci. Technol. 2016, 51, 203–211. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding selectivity of dietary polyphenols to different plant cell wall components: Quantification and mechanism. Food Chem. 2017, 233, 216–227. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Chen, F.; Zhao, G. Effects of molecular structure of polyphenols on their noncovalent interactions with oat β-glucan. J. Agric. Food Chem. 2013, 61, 4533–4538. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Ming, J.; Zhao, G. Optimization of adsorption of tea polyphenols into oat β-glucan using response surface methodology. J. Agric. Food Chem. 2011, 59, 378–385. [Google Scholar] [CrossRef]
- Wu, Z.; Ming, J.; Gao, R.; Wang, Y.; Liang, Q.; Yu, H.; Zhao, G. Characterization and antioxidant activity of the complex of tea polyphenols and oat β-glucan. J. Agric. Food Chem. 2011, 59, 10737–10746. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, J.; Ye, F.; Zhao, G. Non-covalent interaction between ferulic acid and arabinan-rich pectic polysaccharide from rapeseed meal. Int. J Biol. Macromol. 2017, 103, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insight into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material Part I. Effect of some environmental parameters. Biochim. Biophys. Acta 2004, 1672, 192–202. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material. Part II: Quantification and impact of cell wall drying. Biochim. Biophys. Acta 2005, 1725, 1–9. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Li, X.; Cheung, P.C.K. Application of natural β-glucans as biocompatible functional nanomaterials. Food Sci. Hum. Wellness 2019, 8, 315–319. [Google Scholar] [CrossRef]
- Mäkelä, N.; Brinck, O.; Sontag-Strohm, T. Viscosity of β-glucan from oat products at the intestinal phase of the gastrointestinal model. Food Hydrocoll. 2020, 100, 105422. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Dexter, J.E. Barley β-glucans and arabinoxylans: Molecular structure, physicochemical properties and use in food products—A review. Food Res. Int. 2008, 41, 850–868. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P.; Krešić, V.; Barron, A. Adsorption of apple polyphenols onto β-glucan. Czech J. Food Sci. 2017, 35, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Jakobek, L.; Ištuk, J.; Buljeta, I.; Voća, S.; Šic Žlabur, J.; Skendrović Babojelič, S. Traditional, indigenous apple varieties, a fruit with potential for beneficial effects. Their quality traits and bioactive polyphenol content. Foods 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojdylo, A.; Oszmianski, J.; Laskowski, P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Matić, P.; Kraljević, Š.; Ukić, Š.; Benšić, M.; Barron, A.R. Adsorption between quercetin derivatives and β-glucan studied with a novel approach to modeling adsorption isotherms. Appl. Sci. 2020, 10, 1637. [Google Scholar] [CrossRef] [Green Version]
- Soto, M.L.; Moure, A.; Domíniguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption. A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin-Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Marsal, A.; Maldonado, F.; Cuadros, S.; Bautista, M.E.; Manich, A.M. Adsorption isotherm, thermodynamic and kinetics studies of polyphenols onto tannery shaving. Chem. Eng. J. 2012, 183, 21–29. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Lee, K.; Kwong, Y.; Hwang, J.; Choi, Y.; Kim, K.; Koo, H.J.; Seo, Y.; Jeon, H.; Choi, J. Synthesis and functionalization of β-glucan particles for the effective delivery of doxorubicin molecules. ACS Omega 2019, 4, 668–674. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, K.; Gilad, A.A.; Choi, J. Synthesis of beta-glucan nanoparticles for the delivery of single strand DNA. Biotechnol. Bioprocess Eng. 2018, 23, 144–149. [Google Scholar] [CrossRef]
- De Smet, R.; Demoor, T.; Verschuere, S.; Dullaers, M.; Ostroff, G.R.; Leclerq, G.; Allais, L.; Pilette, C.; Dierendonck, M.; De Geest, B.G.; et al. β-glucan microparticles are good candidates for mucosal antigen delivery in oral vaccination. J. Control. Release 2013, 172, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Veverka, M.; Dubaj, T.; Gallovič, J.; Jorik, V.; Veverková, E.; Mičušik, M.; Šimon, P. Beta-glucan complexes with selected nutraceuticals: Synthesis, characterization, and stability. J. Funct. Foods 2014, 8, 309–318. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P. Role of β-glucan in biology of gastrointestinal tract. J. Nat. Sci. 2015, 1, e129. [Google Scholar]




| Polyphenols | Adsorption Capacity | |||||||
|---|---|---|---|---|---|---|---|---|
| mg g−1 | ||||||||
| Peel | Flesh | |||||||
| “Božićnica” | “Batulenka” | “Božićnica” | “Batulenka” | |||||
| Anthocyanins | ||||||||
| cyanidin-3-galactoside | 15.3 ± 0.0 b | |||||||
| Flavan-3-ols | ||||||||
| procyanidin B1 | 109.9 ± 10.8 a,b | 83.2 ± 14.9 c | ||||||
| (+)-catechin | 483.6 ± 42.0 a | |||||||
| (−)-epicatechin | 112.6 ± 16.0 a,b | 48.8 ± 2.7 c,d | ||||||
| Dihydrochalcones | ||||||||
| phloretin-2′-glucoside | 91.4 ± 0.2 a,b | 39.0 ± 5.4 c,d | 5.0 ± 0.5 c | |||||
| phloretin-2′-xyloglucoside | 59.5 ± 2.8 a,b | 13.8 ± 0.7 b,c | ||||||
| Phenolic acids | ||||||||
| chlorogenic acid | 203.3 ± 28.5 a | 61.6 ± 27.0 c,d | 159.7 ± 13.8 a | 19.3 ± 6.3 a | ||||
| chlorogenic acid isomer | 27.5 ± 7.5 a,b | 12.4 ± 2.5 c,d | 34.6 ± 3.8 b | 27.5 ± 8.8 a | ||||
| Flavonols | ||||||||
| quercetin-3-galactoside | 110.0 ± 0.5 a,b | 340.2 ± 44.0 b | ||||||
| quercetin-3 glucoside | 27.6 ± 25.9 a,b | 90.7 ± 8.9 c | ||||||
| quercetin derivative 1 | 16.9 ± 0.5 b | 22.0 ± 1.6 c,d | ||||||
| quercetin derivative 2 | 8.5 ± 4.8 b | 1.3 ± 0.1 d | ||||||
| quercetin-3-xyloside | 12.3 ± 11.8 b | 42.7 ± 4.1 c,d | ||||||
| quercetin-3-rhamnoside | 26.8 ± 3.1 a,b | 71.8 ± 6.6 c,d | 6.3 ± 0.9 c | |||||
| Langmuir | Dubinin–Radushkevich | Hill | ||||||
|---|---|---|---|---|---|---|---|---|
| qm | KL | qs | E | cs | qm | nH | KD | |
| mg g−1 | mg−1 | mg g−1 | J mol−1 | mg | mg g−1 | mg nH | ||
| “Božićnica” | ||||||||
| Anthocyanins | ||||||||
| cyanidin-3-galactoside | 20 | 1163 | 18 | 621 | 0.0007 | 15 | 12 | 0.0005 |
| Flavan-3-ols | ||||||||
| procyanidin B1 | 200 | 117 | 118 | 1438 | 0.0008 | 200 | 2.1 | 0.0069 |
| (−)-epicatechin | 304 | 61 | 126 | 2545 | 0.0150 | 260 | 1.1 | 0.0121 |
| Dihydrochalcones | ||||||||
| phloretin-2′-glucoside | 63 | 291 | 114 | 499 | 0.0008 | 116 | 12.6 | 0.0071 |
| phloretin-2′-xyloglucoside | 60 | 1404 | 54 | 2577 | 0.0020 | 60 | 1.8 | 0.0008 |
| Phenolic acids | ||||||||
| chlorogenic acid | 250 | 175 | 250 | 3237 | 0.0278 | 250 | 1.8 | 0.0059 |
| chlorogenic acid isomer | 50 | 291 | 29 | 1200 | 0.0025 | 50 | 2.3 | 0.0024 |
| Flavonols | ||||||||
| quercetin-3-galactoside | 100 | 110 | 135 | 709 | 0.0150 | 150 | 7.6 | 0.0083 |
| quercetin-3-glucoside | 50 | 261 | 29 | 2862 | 0.0070 | 28 | 4.3 | 0.0015 |
| quercetin derivative 1 | 20 | 1352 | 18 | 1124 | 0.0009 | 20 | 4.8 | 0.0006 |
| quercetin derivative 2 | 20 | 1430 | 14 | 755 | 0.0005 | 20 | 4.2 | 0.0004 |
| quercetin-3-xyloside | 93 | 97 | 13 | 2291 | 0.0022 | 21 | 1.6 | 0.0013 |
| quercetin-3-rhamnoside | 30 | 1075 | 31 | 1181 | 0.0015 | 30 | 4.9 | 0.0010 |
| “Batulenka” | ||||||||
| Flavan-3-ols | ||||||||
| procyanidin B1 | 276 | 498 | 83 | 1703 | 0.0008 | 84 | 3.8 | 0.0003 |
| (+)-catechin | 600 | 515 | 553 | 1144 | 0.0035 | 600 | 4.7 | 0.0021 |
| (−)-epicatechin | 70 | 849 | 70 | 738 | 0.0010 | 70 | 5.5 | 0.0008 |
| Dihydrochalcones | ||||||||
| phloretin-2′-glucoside | 40 | 7820 | 41 | 1688 | 0.0004 | 40 | 3.9 | 0.0002 |
| Phenolic acids | ||||||||
| chlorogenic acid | 75 | 1231 | 74 | 501 | 0.0015 | 75 | 4.8 | 0.0008 |
| chlorogenic acid isomer | 18 | 3594 | 18 | 459 | 0.0002 | 18 | 12.7 | 0.0002 |
| Flavonols | ||||||||
| quercetin-3-galactoside | 500 | 526 | 359 | 1580 | 0.0025 | 500 | 2.7 | 0.0017 |
| quercetin-3-glucoside | 100 | 1225 | 119 | 769 | 0.0010 | 90 | 9.9 | 0.0007 |
| quercetin derivative 1 | 30 | 1553 | 25 | 393 | 0.0005 | 30 | 12.6 | 0.0004 |
| quercetin derivative 2 | 5 | 809 | 5 | 299 | 0.0005 | 5 | 8.6 | 0.0004 |
| quercetin-3-xyloside | 45 | 2125 | 63 | 638 | 0.0006 | 45 | 10.2 | 0.0005 |
| quercetin-3-rhamnoside | 80 | 976 | 80 | 559 | 0.0012 | 80 | 10.7 | 0.0009 |
| Langmuir | Dubinin–Radushkevich | Hill | ||||||
|---|---|---|---|---|---|---|---|---|
| qm | KL | qs | E | cs | qm | nH | KD | |
| mg g−1 | mg−1 | mg g−1 | J mol−1 | mg | mg g−1 | mg nH | ||
| “Božićnica” | ||||||||
| Dihydrochalcones | ||||||||
| phloretin-2′-xyloglucoside | 20 | 3310 | 15 | 998 | 0.0003 | 20 | 3.9 | 0.0002 |
| phloretin-2′-glucoside | 6 | 10,890 | 6 | 687 | 0.0001 | 6 | 10.4 | 0.00009 |
| Phenolic acids | ||||||||
| chlorogenic acid | 200 | 220 | 209 | 655 | 0.0047 | 200 | 9.6 | 0.0034 |
| chlorogenic acid isomer | 50 | 1473 | 43 | 761 | 0.0004 | 50 | 6.1 | 0.0005 |
| Flavonols | ||||||||
| quercetin-3-rhamnoside | 9 | 1634 | 9 | 355 | 0.0006 | 9 | 47 | 0.0006 |
| “Batulenka” | ||||||||
| Phenolic acids | ||||||||
| chlorogenic acid | 60 | 928 | 21 | 2064 | 0.0006 | 27 | 2.2 | 0.0003 |
| chlorogenic acid isomer | 50 | 1002 | 29 | 1584 | 0.0008 | 50 | 2.3 | 0.0007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobek, L.; Buljeta, I.; Ištuk, J.; Barron, A.R. Polyphenols of Traditional Apple Varieties in Interaction with Barley β-Glucan: A Study of the Adsorption Process. Foods 2020, 9, 1278. https://doi.org/10.3390/foods9091278
Jakobek L, Buljeta I, Ištuk J, Barron AR. Polyphenols of Traditional Apple Varieties in Interaction with Barley β-Glucan: A Study of the Adsorption Process. Foods. 2020; 9(9):1278. https://doi.org/10.3390/foods9091278
Chicago/Turabian StyleJakobek, Lidija, Ivana Buljeta, Jozo Ištuk, and Andrew R. Barron. 2020. "Polyphenols of Traditional Apple Varieties in Interaction with Barley β-Glucan: A Study of the Adsorption Process" Foods 9, no. 9: 1278. https://doi.org/10.3390/foods9091278