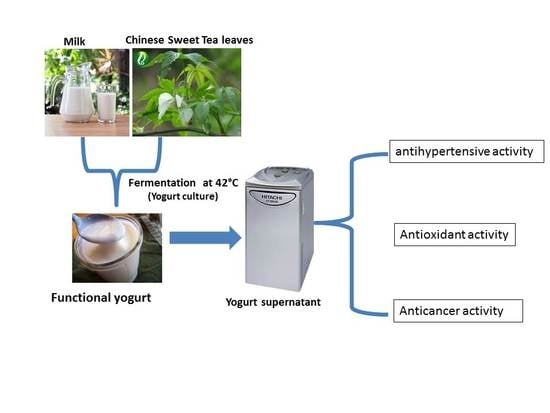
Development of a Multifunction Set Yogurt Using Rubus suavissimus S. Lee (Chinese Sweet Tea) Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Chinese Sweet Tea Extract
2.3. High-Performance Liquid Chromatography (HPLC) Analysis of Phenolic Compounds
2.4. Yogurt Manufacture
2.5. Chemical Composition
2.6. Bacterial Counts
2.7. Yogurt Supernatant
2.8. Total Phenolic Content (TPC)
2.9. Antioxidant Activity
2.10. Anticancer Activity
2.11. Antihypertensive Activity
2.12. Sensory Evaluation
2.13. Statistical Analysis
3. Results and Discussion
3.1. Impact of the Chinese Sweet Tea Extract on the Chemical Composition of the Yogurt Samples
3.2. Identification and Quantification of Phenolic Compounds in the Chinese Sweet Tea Extract
3.3. pH and Bacterial Count
3.4. Antioxidant Activity
3.5. Anticancer Activity
3.6. Antihypertensive Activity
3.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khoshnoudi-Nia, S.; Sharif, N.; Jafari, S.M. Loading of phenolic compounds into electrospun nanofibers and electrosprayed nanoparticles. Trends Food Sci. Technol. 2020, 95, 59–74. [Google Scholar] [CrossRef]
- Chou, G.; Xu, S.-J.; Liu, D.; Koh, G.Y.; Zhang, J.; Liu, Z. Quantitative and fingerprint analyses of Chinese sweet tea plant (Rubus suavissimus S. Lee). J. Agric. Food Chem. 2009, 57, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- Koh, G.Y.; Chou, G.; Liu, Z. Purification of a water extract of Chinese sweet tea plant (Rubus suavissimus S. lee) by alcohol precipitation. J. Agric. Food Chem. 2009, 57, 5000–5006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezure, T.; Amano, S. Rubus suavissimus S. Lee extract increases early adipogenesis in 3T3-L1 preadipocytes. J. Nat. Med. 2011, 65, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.; Liu, Q.; Xie, S.; Chen, M.; Wang, L.; Feng, Y.; Chen, X. Comprehensive profiling of α-glucosidase inhibitors from the leaves of Rubus suavissimus using an off-line hyphenation of HSCCC, ultrafiltration HPLC-UV-MS and prep-HPLC. J. Food Compos. Anal. 2020, 85, 103336. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, R.; Zeng, Y.; Tsao, R.; Mine, Y. Chinese sweet leaf tea (Rubus suavissimus) mitigates LPS-induced low-grade chronic inflammation and reduces the risk of metabolic disorders in a C57BL/6J mouse model. J. Agric. Food Chem. 2020, 68, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic fortification of yogurt using grape and callus extracts. LWT—Food Sci. Technol. 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Zhang, T.; Jeong, C.H.; Cheng, W.N.; Bae, H.; Seo, H.G.; Petriello, M.C.; Han, S.G. Moringa extract enhances the fermentative, textural, and bioactive properties of yogurt. LWT—Food Sci. Technol. 2019, 101, 276–284. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT—Food Sci. Technol. 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Jaziri, I.; Ben Slama, M.; Mhadhbi, H.; Urdaci, M.C.; Hamdi, M. Effect of green and black teas (Camellia sinensis L.) on the characteristic microflora of yogurt during fermentation and refrigerated storage. Food Chem. 2009, 112, 614–620. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Sady, M.; Grega, T.; Walczycka, M. The impact of tea supplementation on microflora, pH and antioxidant capacity of yoghurt. Int. Dairy J. 2011, 21, 568–574. [Google Scholar] [CrossRef]
- Amirdivani, S.; Baba, A.S.H. Green tea yogurt: Major phenolic compounds and microbial growth. J. Food Sci. Technol. 2015, 52, 4652–4660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT—Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Huang, Z.; Enomoto, T.; Huang, L.; Li, L. Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chem. 2020, 303, 125400. [Google Scholar] [CrossRef]
- Amirdivani, S.; Baba, A.S. Changes in yogurt fermentation characteristics, and antioxidant potential and in vitro inhibition of angiotensin-1 converting enzyme upon the inclusion of peppermint, dill and basil. LWT—Food Sci. Technol. 2011, 44, 1458–1464. [Google Scholar] [CrossRef] [Green Version]
- Ramchandran, L.; Shah, N.P. Growth, proteolytic, and ACE-I activities of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus and rheological properties of low-fat yogurt as influenced by the addition of Raftiline HP®. J. Food Sci. 2008, 73, 368–374. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Antibacterial and antiproliferative peptides in synbiotic yogurt-Release and stability during refrigerated storage. J. Dairy Sci. 2016, 99, 4233–4242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Washington, DC, USA, 2000.
- Türköz Acar, E.; Celep, M.E.; Charehsaz, M.; Akyüz, G.S.; Yeşilada, E. Development and validation of a high-performance liquid chromatography–diode-array detection method for the determination of eight phenolic constituents in extracts of different wine species. Turk. J. Pharm. Sci. 2018, 15, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Romeih, E.; Gamba, R.R.; Nagai, E.; Suzuki, T.; Koyanagi, T.; Enomoto, T. The biological activity of fermented milk produced by Lactobacillus casei ATCC 393 during cold storage. Int. Dairy J. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Romeih, E.A.; Abdel-Hamid, M.; Awad, A.A. The addition of buttermilk powder and transglutaminase improves textural and organoleptic properties of fat-free buffalo yogurt. Dairy Sci. Technol. 2014, 94, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.-X.; Yu, Z.-L.; He, Q.; Tang, S.-H.; Zeng, W.-C. A potentially functional yogurt co-fermentation with Gnaphalium affine. LWT—Food Sci. Technol. 2018, 91, 423–430. [Google Scholar] [CrossRef]
- Behrad, S.; Yusof, M.Y.; Goh, K.L.; Baba, A.S. Manipulation of probiotics fermentation of yogurt by cinnamon and licorice: Effects on yogurt formation and inhibition of Helicobacter pylori growth in vitro. World Acad. Sci. Eng. Technol. 2009, 36, 590–594. [Google Scholar] [CrossRef]
- Illupapalayam, V.V.; Smith, S.C.; Gamlath, S. Consumer acceptability and antioxidant potential of probiotic-yogurt with spices. LWT—Food Sci. Technol. 2014, 55, 255–262. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.M.C.; Coelho, M.; Lopes, C.; Almeida, D.P.F.; Pintado, M. Incorporation of strawberries preparation in yoghurt: Impact on phytochemicals and milk proteins. Food Chem. 2015, 171, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B.; Rashid, F.; Baba, A.S. Effect of the addition of phytomix-3+ mangosteen on antioxidant activity, viability of lactic acid bacteria, type 2 diabetes key-enzymes, and sensory evaluation of yogurt. LWT—Food Sci. Technol. 2018, 94, 33–39. [Google Scholar] [CrossRef]
- George Thompson, A.M.; Iancu, C.V.; Nguyen, T.T.H.; Kim, D.; Choe, J. Inhibition of human GLUT1 and GLUT5 by plant carbohydrate products; insights into transport specificity. Sci. Rep. 2015, 5, 12804. [Google Scholar] [CrossRef] [Green Version]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.M.; Qi, X.W.; Hao, J.H.; Liu, H.F.; Xu, Q.H.; Bu, P.L. In vitro, ex vivo and in vivo anti-hypertensive activity of Chrysophyllum cainito L. Extract. Int. J. Clin. Exp. Med. 2015, 8, 17912–17921. [Google Scholar]
- Balasuriya, N.; Rupasinghe, H.P.V. Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem. 2012, 135, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Lee, J.; Lee, W.; Ko, J.; Kim, E.; Kim, J.; Heu, M.; Hoon, G.; Jeon, Y. Gallic acid isolated from Spirogyra sp. improves cardiovascular disease through a vasorelaxant and antihypertensive effect. Environ. Toxicol. Pharmacol. 2015, 39, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Shaw, H.; Wu, J.; Wang, M. Antihypertensive effects of Ocimum gratissimum extract: Angiotensin-converting enzyme inhibitor in vitro and in vivo investigation. J. Funct. Foods 2017, 35, 68–73. [Google Scholar] [CrossRef]
- Zhu, X.H.; Xia, M.; Yang, C.X. Optimizing the Extraction Process of Rubusoside from the Rubus Suavissimus: A Cellulase Pretreatment Approach. Appl. Mech. Mater. 2014, 707, 144–148. [Google Scholar] [CrossRef]
- Liu, R.; Finley, J. Potential cell culture models for antioxidant research. J. Agric. Food Chem. 2005, 53, 4311–4314. [Google Scholar] [CrossRef]
| Characteristic | Chinese Sweet Tea Extract |
|---|---|
| Total solid (%) | 94.22 ± 1.0 |
| Protein (%) | 6.40 ± 0.25 |
| Fat (%) | 0.42 ± 0.1 |
| Ash (%) | 7.21 ± 0.1 |
| Carbohydrates (%) | 80.18 ± 1.2 |
| Total phenolic content * | 21.54 ± 0.5 |
| ACE-I activity (%) | 86.85 ± 2.1 |
| Anticancer activity (%) | 89.06 ± 3.7 |
| Chinese Sweet Tea Extract Concentration | Protein (%) | Carbohydrates (%) | Total Solids (%) | Total Phenolic Content * |
|---|---|---|---|---|
| 0% (Control) | 5.10 ± 0.08 A | 6.17 ± 0.01 D | 12.31 ± 0.02 D | 11.64 ± 1.2 D |
| 0.25% | 5.15 ± 0.02 A | 6.26 ± 0.02 C | 12.56 ± 0.02 C | 41.60 ± 2.0 C |
| 0.5% | 5.16 ± 0.02 A | 6.35 ± 0.01 B | 12.84 ± 0.04 B | 70.93 ± 2.2 B |
| 1% | 5.18 ± 0.02 A | 6.59 ± 0.01 A | 13.48 ± 0.02 A | 130.58 ± 2.2 A |
| Phenolics | Concentration (mg/g) |
|---|---|
| Gallic acid | 1.26 ± 0.006 |
| Catchin | 0.08 ± 0.001 |
| Chlorgenic | 0.66 ± 0.003 |
| Vanillic acid | 0.96 ± 0.001 |
| Caffeic acid | 0.47 ± 0.005 |
| Syringic acid | 1.53 ± 0.006 |
| p-Coumaric acid | 1.08 ± 0.006 |
| Benzoic acid | 2.77 ± 0.007 |
| Ferulic acid | 0.11 ± 0.001 |
| Rutin | 1.63 ± 0.004 |
| Ellagic | 1.44 ± 0.007 |
| o-Coumaric acid | 0.74 ± 0.004 |
| Resveratrol | 0.52 ± 0.003 |
| Cinnamic acid | 0.11 ± 0.001 |
| Quercetin | 1.98 ± 0.005 |
| Neringein | 0.59 ± 0.004 |
| Rosemarinic | 0.55 ± 0.005 |
| Myricetin | 0.46 ± 0.005 |
| Kampherol | 0.38 ± 0.004 |
| Chinese Sweet Tea Extract Concentration | pH | L. bulgaricus (log CFU/g) | S. thermophilus (log CFU/g) |
|---|---|---|---|
| 0% (Control) | 4.52 ± 0.02 A | 8.65 ± 0.1 A | 9.20 ± 0.04 A |
| 0.25% | 4.53 ± 0.02 A | 8.83 ± 0.03 A | 9.22 ± 0.03 A |
| 0.5% | 4.54 ± 0.03 A | 8.90 ± 0.04 A | 9.25 ± 0.1 A |
| 1% | 4.54 ± 0.02 A | 8.86 ± 0.5 A | 9.18 ± 0.02 A |
| Chinese Sweet Tea Extract Concentration | Antioxidant Activity (%) | |
|---|---|---|
| ABTS | DPPH | |
| 0% (Control) | 32.01 ± 1.0 D | 14.20 ± 0.4 D |
| 0.25% | 49.40 ± 0.9 C | 29.85 ± 1.4 C |
| 0.5% | 68.76 ± 2.2 B | 47.29 ± 2.2 B |
| 1% | 92.43 ± 0.3 A | 74.83 ± 0.9 A |
| Sweet Tea Extract Concentration | ACE-I (%) | Antiproliferative Activity (%) |
|---|---|---|
| 0% (Control) | 44.20 ± 0.7 D | 18.58 ± 1.4 C |
| 0.25% | 50.88 ± 1.1 C | 26.27 ± 4.5 C |
| 0.5% | 64.72 ± 1.6 B | 52.41 ± 6.1 B |
| 1% | 82.03 ± 1.9 A | 67.46 ± 1.9 A |
| Chinese Sweet Tea Extract Concentration | Appearance | Aroma | Texture | Overall Acceptability |
|---|---|---|---|---|
| 0% (Control) | 7.7 ± 0.5 A | 6.9 ± 0.4 C | 6.0 ± 0.8 C | 6.7 ± 0.8 C |
| 0.25% | 8.0 ± 0.7 A | 7.7 ± 0.5 B | 6.6 ± 0.5 C | 7.6 ± 0.5 B |
| 0.5% | 8.1 ± 0.8 A | 8.6 ± 0.5 A | 7.6 ± 0.5 B | 8.7 ± 0.5 A |
| 1% | 8.3 ± 0.8 A | 6.0 ± 0.6 D | 8.7 ± 0.5 A | 5.7 ± 0.5 D |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Hamid, M.; Huang, Z.; Suzuki, T.; Enomoto, T.; Hamed, A.M.; Li, L.; Romeih, E. Development of a Multifunction Set Yogurt Using Rubus suavissimus S. Lee (Chinese Sweet Tea) Extract. Foods 2020, 9, 1163. https://doi.org/10.3390/foods9091163
Abdel-Hamid M, Huang Z, Suzuki T, Enomoto T, Hamed AM, Li L, Romeih E. Development of a Multifunction Set Yogurt Using Rubus suavissimus S. Lee (Chinese Sweet Tea) Extract. Foods. 2020; 9(9):1163. https://doi.org/10.3390/foods9091163
Chicago/Turabian StyleAbdel-Hamid, Mahmoud, Zizhen Huang, Takuya Suzuki, Toshiki Enomoto, Ahmed M. Hamed, Ling Li, and Ehab Romeih. 2020. "Development of a Multifunction Set Yogurt Using Rubus suavissimus S. Lee (Chinese Sweet Tea) Extract" Foods 9, no. 9: 1163. https://doi.org/10.3390/foods9091163