Effect of Physical Structures of Food Matrices on Heat Resistance of Enterococcus faecium NRRL-2356 in Wheat Kernels, Flour and Dough
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Characteristics
2.3. Preparation and Inoculation of Cell Suspension
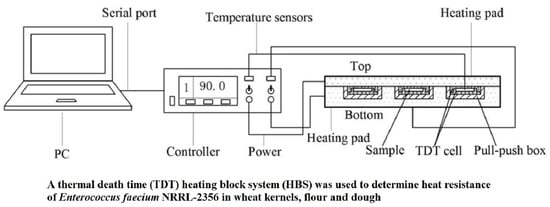
2.4. Heat Treatment
2.4.1. Comparison of Microbial Heat Resistances among E. faecium, Salmonella Cocktail, and E. coli
2.4.2. Thermal Inactivation Kinetics
2.5. Enumeration
2.6. Fitting of Kinetic Inactivation
2.7. Microstructure Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Selection of the Most Heat-Resistant Strain in Wheat Kernels and Flour
3.2. Influence of Graininess and Powder Structures of Food Matrices on Heat Resistance of E. faecium
3.3. Influence of Graininess and Paste Structures of Food Matrices on Heat Resistance of E. faecium
3.4. Microstructure Observation by SEM
3.5. Influence of Physical Structures of Food Matrices on Heat Resistance of E. faecium
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Forghani, F.; den Bakker, M.; Liao, J.Y.; Payton, A.S.; Futral, A.N.; Diez-Gonzalez, F. Salmonella and enterohemorrhagic Escherichia coli serogroups O45, O121, O145 in wheat flour: Effects of long-term storage and thermal treatments. Front. Microbiol. 2019, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.G.; Kang, D.H. Inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in ready-to-bake cookie dough by gamma and electron beam irradiation. Food Microbiol. 2017, 64, 172–178. [Google Scholar] [CrossRef] [PubMed]
- McCallum, L.; Paine, S.; Sexton, K.; Dufour, M.; Dyet, K.; Wilson, M.; Campbell, D.; Bandaranayake, D.; Hope, V. An outbreak of Salmonella Typhimurium phage type 42 associated with the consumption of raw flour. Foodborne Pathog. Dis. 2013, 10, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Myoda, S.P.; Gilbreth, S.; Akins-Leventhal, D.; Davidson, S.K.; Samadpour, M. Occurrence and levels of Salmonella, enterohemorrhagic Escherichia coli, and Listeria in raw wheat. J. Food Prot. 2019, 82, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Berghofer, L.K.; Hocking, A.D.; Miskelly, D.; Jansson, E. Microbiology of wheat and flour milling in Australia. Int. J. Food Microbiol. 2003, 85, 137–149. [Google Scholar] [CrossRef]
- Sabillon, L.; Bianchini, A. From field to table: A review on the microbiological quality and safety of wheat-based products. Cereal Chem. 2016, 93, 105–115. [Google Scholar] [CrossRef]
- Channaiah, L.H.; Michael, M.; Acuff, J.C.; Phebus, R.K.; Thippareddi, H.; Milliken, G. Evaluation of thermal inactivation parameters of Salmonella in whole wheat multigrain bread. Food Microbiol. 2019, 82, 334–341. [Google Scholar] [CrossRef]
- Wu, S.; Ricke, S.C.; Schneider, K.R.; Ahn, S. Food safety hazards associated with ready-to-bake cookie dough and its ingredients. Food Control. 2017, 73, 986–993. [Google Scholar] [CrossRef]
- CDC. Notes from the Field: Multistate Outbreak of Escherichia coli O157:H7 Infections Linked to Dough Mix-United States. 2017. Available online: https://www.cdc.gov/mmwr/volumes/66/wr/mm6603a6.htm?s_cid=mm6603a6_w (accessed on 15 May 2020).
- CDC. Multistate Outbreak of Shiga Toxin-Producing Escherichia coli Infections Linked to Flour. 2016. Available online: https://www.cdc.gov/ecoli/2016/o121-06-16/index.html (accessed on 15 May 2020).
- Hildebrandt, I.M.; Marks, B.P.; Ryser, E.T.; Villa-Rojas, R.; Tang, J.M.; Garces-Vega, F.J.; Buchholz, S.E. Effects of inoculation procedures on variability and repeatability of Salmonella thermal resistance in wheat flour. J. Food Prot. 2016, 79, 1833–1839. [Google Scholar] [CrossRef]
- Limcharoenchat, P.; Buchholz, S.E.; James, M.K.; Hall, N.O.; Ryser, E.T.; Marks, B.P. Inoculation protocols influence the thermal resistance of Salmonella Enteritidis PT 30 in fabricated almond, wheat, and date products. J. Food Prot. 2018, 81, 606–613. [Google Scholar] [CrossRef] [Green Version]
- Syamaladevi, R.M.; Tadapaneni, R.K.; Xu, J.; Villa-Rojas, R.; Tang, J.M.; Carter, B.; Sablani, S.; Marks, B.P. Water activity change at elevated temperatures and thermal resistance of Salmonella in all purpose wheat flour and peanut butter. Food Res. Int. 2016, 81, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Tang, J.M.; Jin, Y.Q.; Song, J.X.; Yang, R.; Sablani, S.S.; Zhu, M.J. High temperature water activity as a key factor influencing survival of Salmonella Enteritidis PT30 in thermal processing. Food Control 2019, 98, 520–528. [Google Scholar] [CrossRef]
- Zhang, L.H.; Kou, X.X.; Zhang, S.; Cheng, T.; Wang, S.J. Effect of water activity and heating rate on Staphylococcus aureus heat resistance in walnut shells. Int. J. Food Microbiol. 2018, 266, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Perez-Baltar, A.; Alia, A.; Rodriguez, A.; Cordoba, J.J.; Medina, M.; Montiel, R. Impact of water activity on the inactivation and gene expression of Listeria monocytogenes during refrigerated storage of pressurized dry-cured ham. Foods 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.G.; Kang, D.H. Influence of moisture content on inactivation of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in powdered red and black pepper spices by radio-frequency heating. Int. J. Food Microbiol. 2014, 176, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Rojas, R.V.; Gray, P.; Zhu, M.J.; Tang, J.M. Enterococcus faecium as a Salmonella surrogate in the thermal processing of wheat flour: Influence of water activity at high temperatures. Food Microbiol. 2018, 74, 92–99. [Google Scholar] [CrossRef]
- Smith, D.F.; Hildebrandt, I.M.; Casulli, K.E.; Dolan, K.D.; Marks, B.P. Modeling the effect of temperature and water activity on the thermal resistance of Salmonella Enteritidis PT 30 in wheat flour. J. Food Prot. 2016, 79, 2058–2065. [Google Scholar] [CrossRef]
- Zhang, B.H.; Zhang, L.H.; Cheng, T.; Guan, X.Y.; Wang, S.J. Effects of water activity, temperature and particle size on thermal inactivation of Escherichia coli ATCC 25922 in red pepper powder. Food Control 2020, 107, 106817. [Google Scholar] [CrossRef]
- Xu, N.N.; Wang, D.M.; Liu, J.X. Variance of zein protein and starch granule morphology between corn and steam flaked products determined starch ruminal degradability through altering starch hydrolyzing bacteria attachment. Anim. 2019, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Quinto, E.J.; Marin, J.M.; Caro, I.; Mateo, J.; Schaffner, D.W. Modelling growth and decline in a two-species model system: Pathogenic Escherichia coli O157:H7 and psychrotrophic spoilage bacteria in milk. Foods 2020, 9, 331. [Google Scholar] [CrossRef] [Green Version]
- Villa-Rojas, R.; Tang, J.; Wang, S.J.; Gao, M.X.; Kang, D.H.; Mah, J.H.; Gray, P.; Sosa-Morales, M.E.; Lopez-Malo, A. Thermal inactivation of Salmonella Enteritidis PT 30 in almond kernels as influenced by water activity. J. Food Prot. 2013, 76, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Jeon, E.B.; Choi, M.S.; Choi, E.H.; Lim, J.S.; Choi, J.; Park, S.Y. The efficiency of atmospheric dielectric barrier discharge plasma against Escherichia coli and Bacillus cereus on dried laver (Porphyra tenera). Foods 2020, 9, 1013. [Google Scholar] [CrossRef] [PubMed]
- Garre, A.; Fernandez, P.S.; Lindqvist, R.; Egea, J.A. Bioinactivation: Software for modelling dynamic microbial inactivation. Food Res. Int. 2017, 93, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.H. Thermal inactivation of Listeria monocytogenes in ground beef under isothermal and dynamic temperature conditions. J. Food Eng. 2009, 90, 380–387. [Google Scholar] [CrossRef]
- Ozturk, S.; Kong, F.B.; Singh, R.K. Evaluation of Enterococcus faecium NRRL B-2354 as a potential surrogate of Salmonella in packaged paprika, white pepper and cumin powder during radio frequency heating. Food Control 2020, 108, 106833. [Google Scholar] [CrossRef]
- Van Boekel, M. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int. J. Food Microbiol. 2002, 74, 139–159. [Google Scholar] [CrossRef]
- Kou, X.X.; Li, R.; Hou, L.X.; Huang, Z.; Ling, B.; Wang, S.J. Performance of a heating block system designed for studying the heat resistance of bacteria in foods. Sci. Rep. 2016, 6, 30758. [Google Scholar] [CrossRef]
- Kou, X.X.; Li, R.; Hou, L.X.; Cheng, T.; Zhou, X.; Wang, S.J. Evaluation of a heating block system for accurate temperature-time controlled pasteurization treatments on various foods. Int. J. Agric. Biol. Eng. 2018, 11, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Kou, X.X.; Cheng, T.; Zheng, A.J.; Wang, S.J. Verification of radio frequency pasteurization process for in-shell almonds. J. Food Eng. 2017, 192, 103–110. [Google Scholar] [CrossRef]
- Liu, S.X.; Ozturk, S.; Xu, J.; Kong, F.B.; Gray, P.; Zhu, M.J.; Sablani, S.S.; Tang, J.M. Microbial validation of radio frequency pasteurization of wheat flour by inoculated pack studies. J. Food Eng. 2018, 217, 68–74. [Google Scholar] [CrossRef]
- Wei, X.Y.; Lau, S.K.; Stratton, J.; Irmak, S.; Subbiah, J. Radiofrequency pasteurization process for inactivation of Salmonella spp. and Enterococcus faecium NRRL B-2354 on ground black pepper. Food Microbiol. 2019, 82, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Brar, P.K.; Danyluk, M.D. Validation of Enterococcus faecium as a surrogate for Salmonella under different processing conditions for peanuts and pecans. Food Microbiol. 2019, 80, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Eblen, D.R.; Annous, B.A.; Sapers, G.M. Studies to select appropriate nonpathogenic surrogate Escherichia coli strains for potential use in place of Escherichia coli O157: H7 and Salmonella in pilot plant studies. J. Food Prot. 2005, 68, 282–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simsek, S.; Snelling, J.; Malekmohammadi, S.; Bergholz, T.M. Vacuum steam treatment of soft wheat: Quality and reduction of Escherichia coli O121 and Salmonella Enteritidis PT30. Cereal Chem. 2020. [Google Scholar] [CrossRef]
- Boreddy, S.R.; Rose, D.J.; Subbiah, J. Radiofrequency-Assisted Thermal Processing of Soft Wheat Flour. J. Food Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Tiwari, G.; Tang, J.; Wang, S. Frequency, moisture and temperature-dependent dielectric properties of chickpea flour. Biosyst. Eng. 2008, 101, 217–224. [Google Scholar] [CrossRef]
- Bansal, N.; Dhaliwal, A.S.; Mann, K.S. Dielectric properties of corn flour from 0.2 to 10 GHz. J. Food Eng. 2015, 166, 255–262. [Google Scholar] [CrossRef]
- Taylor, M.H.; Tsai, H.C.; Rasco, B.; Tang, J.M.; Zhu, M.J. Stability of Listeria monocytogenes in wheat flour storage and isothermal treatment. Food Control 2018, 91, 434–439. [Google Scholar] [CrossRef]
- Zhang, L.; Lyng, J.G.; Xu, R.; Zhang, S.; Zhou, X.; Wang, S. Influence of radio frequency treatment on in-shell walnut quality and Staphylococcus aureus ATCC 25923 survival. Food Control 2019, 102, 197–205. [Google Scholar] [CrossRef]
- Huang, L. Reconciliation of the D/z model and the Arrhenius model: The effect of temperature on inactivation rates of chemical compounds and microorganisms. Food Chem. 2019, 295, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Couvert, O.; Gaillard, S.; Savy, N.; Mafart, P.; Leguerinel, I. Survival curves of heated bacterial spores: Effect of environmental factors on Weibull parameters. Int. J. Food Microbiol. 2005, 101, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwietering, M.H. Modelling Microorganisms in Food, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 110–125. [Google Scholar]
- Huang, L.H. IPMP 2013-a comprehensive data analysis tool for predictive microbiology. Int. J. Food Microbiol. 2014, 171, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Collado, J.; Cunha, L.M.; Ocio, M.J.; Martinez, A. Empirical model building based on Weibull distribution to describe the joint effect of pH and temperature on the thermal resistance of Bacillus cereus in vegetable substrate. Int. J. Food Microbiol. 2002, 77, 147–153. [Google Scholar] [CrossRef]
- Fernandez, A.; Lopez, M.; Bernardoa, A.; Condon, S.; Raso, J. Modelling thermal inactivation of Listeria monocytogenes in sucrose solutions of various water activities. Food Microbiol. 2007, 24, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Baril, E.; Coroller, L.; Postollec, F.; Leguerinel, I.; Boulais, C.; Carlin, F.; Mafart, P. The wet-heat resistance of Bacillus weihenstephanensis KBAB4 spores produced in a two-step sporulation process depends on sporulation temperature but not on previous cell history. Int. J. Food Microbiol. 2011, 146, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.Y.; Wang, S.J. Dielectric properties, heating rate, and heating uniformity of wheat flour with added bran associated with radio frequency treatments. Innovative Food Sci. Emerg. Technol. 2020, 60, 102290. [Google Scholar] [CrossRef]
- Hu, Y.M.; Nie, W.; Hu, X.Z.; Li, Z.G. Microbial decontamination of wheat grain with superheated steam. Food Control. 2016, 62, 264–269. [Google Scholar] [CrossRef]
- Sabillon, L.; Stratton, J.; Rose, D.; Bianchini, A. Reduction in pathogenic load of wheat by tempering with saline organic acid solutions at different seasonal temperatures. Int. J. Food Microbiol. 2020, 313, 108381. [Google Scholar] [CrossRef]






| Food Matrices | Dry Wheat Kernels | Wheat Flour | Wet Wheat Kernels | Wheat Dough |
|---|---|---|---|---|
| MC (% w.b.) | 11.25 ± 0.09 a 1 | 11.39 ± 0.09 a | 38.45 ± 0.04 b | 38.54 ± 0.07 b |
| aw at 25 °C | 0.66 ± 0.01 a | 0.65 ± 0.01 a | 0.99 ± 0.01 b | 0.99 ± 0.01 b |
| Density in TDT cell (g/mL) | 1.33 ± 0.03 a | 0.71 ± 0.02 b | 1.27 ± 0.01 c | 0.91 ± 0.03 d |
| Inoculation concentration (log CFU/g) | 8.83 ± 0.20 a | 9.23 ± 0.53 a | 9.37 ± 0.15 a | 9.31 ± 0.16 a |
| z-value (°C) | 9.33 ± 0.81 a | 10.71 ± 0.60 b | 4.94 ± 0.04 c | 5.64 ± 0.25 d |
| δ’75 °C (min) 2 | 13.91 ± 1.08 A | 17.67 ± 0.85 B | ||
| δ’80 °C (min) | 4.13 ± 0.12 A | 6.30 ± 0.39 B | ||
| δ’85 °C (min) | 1.12 ± 0.12 A | 2.41 ± 0.18 B | ||
| δ’60 °C (min) | 6.86 ± 0.54 A | 14.90 ± 0.70 B | ||
| δ’62 °C (min) | 4.28 ± 0.49 A | 6.20 ± 0.32 B | ||
| δ’64 °C (min) | 1.07 ± 0.11 A | 2.85 ± 0.11 B |
| Material | Temperature (°C) | Linear Model | Weibull Model | |||||
|---|---|---|---|---|---|---|---|---|
| D-Value (min) | RMSE (log CFU/g) | AIC | δ (min) | p | RMSE (log CFU/g) | AIC | ||
| Wheat kernels | 75 | 14.04 ± 0.78 | 0.27 | −33.461 | 16.72 ± 2.56 | 1.15 ± 0.15 | 0.27 | −30.894 |
| 80 | 4.56 ± 0.35 | 0.32 | −28.485 | 3.31 ± 0.86 | 0.79 ± 0.13 | 0.31 | −26.998 | |
| 85 | 1.22 ± 0.09 | 0.39 | −22.437 | 1.06 ± 0.29 | 0.91 ± 0.15 | 0.40 | −18.784 | |
| Wheat flour | 75 | 18.59 ± 1.49 | 0.38 | −23.014 | 17.13 ± 4.71 | 0.94 ± 0.18 | 0.40 | −19.147 |
| 80 | 6.49 ± 0.56 | 0.44 | −19.133 | 6.45 ± 1.89 | 0.99 ± 0.20 | 0.45 | −15.134 | |
| 85 | 2.08 ± 0.21 | 0.40 | −21.498 | 3.28 ± 0.41 | 1.75 ± 0.32 | 0.31 | −26.794 | |
| Material | Temperature (°C) | Linear Model | Weibull Model | |||||
|---|---|---|---|---|---|---|---|---|
| D-Value (min) | RMSE (log CFU/g) | AIC | δ (min) | p | RMSE (log CFU/g) | AIC | ||
| Wheat kernels | 60 | 6.84 ± 0.48 | 0.48 | −16.172 | 7.22 ± 2.00 | 1.03 ± 0.17 | 0.50 | −12.235 |
| 62 | 4.33 ± 0.23 | 0.34 | −26.343 | 4.45 ± 0.92 | 1.02 ± 0.13 | 0.36 | −22.371 | |
| 64 | 1.13 ± 0.08 | 0.41 | −21.149 | 1.00 ± 0.28 | 0.93 ± 0.15 | 0.42 | −17.431 | |
| Wheat dough | 60 | 18.04 ± 1.39 | 0.40 | −21.758 | 6.59 ± 1.48 | 0.59 ± 0.04 | 0.20 | −39.236 |
| 62 | 7.16 ± 0.66 | 0.52 | −13.509 | 3.52 ± 1.44 | 0.68 ± 0.12 | 0.46 | −14.930 | |
| 64 | 3.23 ± 0.33 | 0.57 | −11.019 | 1.82 ± 0.84 | 0.72 ± 0.16 | 0.54 | −9.944 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, B.; Zhu, Y.; Zhang, L.; Xu, R.; Guan, X.; Kou, X.; Wang, S. Effect of Physical Structures of Food Matrices on Heat Resistance of Enterococcus faecium NRRL-2356 in Wheat Kernels, Flour and Dough. Foods 2020, 9, 1890. https://doi.org/10.3390/foods9121890
Lin B, Zhu Y, Zhang L, Xu R, Guan X, Kou X, Wang S. Effect of Physical Structures of Food Matrices on Heat Resistance of Enterococcus faecium NRRL-2356 in Wheat Kernels, Flour and Dough. Foods. 2020; 9(12):1890. https://doi.org/10.3390/foods9121890
Chicago/Turabian StyleLin, Biying, Yufei Zhu, Lihui Zhang, Ruzhen Xu, Xiangyu Guan, Xiaoxi Kou, and Shaojin Wang. 2020. "Effect of Physical Structures of Food Matrices on Heat Resistance of Enterococcus faecium NRRL-2356 in Wheat Kernels, Flour and Dough" Foods 9, no. 12: 1890. https://doi.org/10.3390/foods9121890