Rice Flour and Bran Enriched with Blueberry Polyphenols Increases Storage Stability and Decreases Arsenic Content in Bran
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
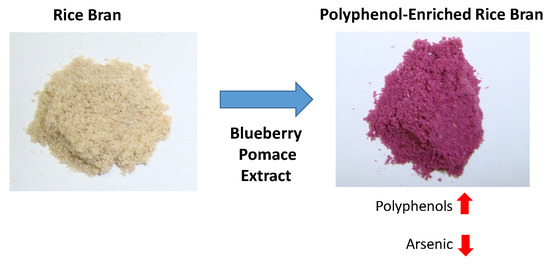
2.2. Preparation of Rice Blueberry Complexes
2.3. Polyphenol and Anthocyanin Analysis
2.4. Storage Stability of Polyphenols and Anthocyanins
2.5. Colorimetric Parameters
2.6. Glucose, Fructose, and Sucrose Analysis
2.7. Arsenic Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Blueberry Juice Production and Pomace Extractions
3.2. Polyphenol and Anthocyanin Content of Blueberry-Enriched Rice Flour and Bran
3.3. Changes in Sugar Composition
3.4. Changes in Polyphenols and Anthocyanins during Storage
3.5. Changes in Color Parameters during Storage
3.6. Total and Inorganic Arsenic Content in Rice Bran
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fasano, A.; Catassi, C. Current approaches to diagnosis and treatment of celiac disease: An evolving spectrum. Gastroenterology 2001, 120, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.M. The properties of rice bran as a food stuff. Cereal Foods World 1990, 35, 632–662. [Google Scholar]
- Friedman, M. Rice brans, rice bran oils, and rice hulls: Composition, food and industrial uses, and bioactivities in humans, animals, and cells. J. Agric. Food Chem. 2013, 61, 10626–10641. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Kleisiaris, F.; Kadoglidou, K.; Katsantonis, D. Optimizing extraction conditions of free and bound phenolic compounds from rice by-products and their antioxidant effects. Foods 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Shih, F. An update on the use of co-products from the milling of rice in value-added food products. J. Am. Oil Chem. Soc. 2012, 89, 1–8. [Google Scholar] [CrossRef]
- Meharg, A.A.; Lombi, E.; Williams, P.N.; Scheckel, K.G.; Feldmann, J.; Raab, A.; Zhu, Y.; Islam, R. Speciation and localization of arsenic in white and brown rice grains. Environ. Sci. Technol. 2008, 42, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- US-FDA (US Food and Drug Administration). Arsenic in Rice and Rice Products Risk Assessment Report. 2016. Available online: http://www.fda.gov/downloads/food/foodscienceresearch/risksafetyassessment/ucm486543.pdf (accessed on 22 May 2019).
- Xu, X.Y.; McGrath, S.P.; Meharg, A.; Zhao, F.J. Growing rice aerobically markedly decreases arsenic accumulation. Environ. Sci. Technol. 2008, 42, 5574–5579. [Google Scholar] [CrossRef]
- Talukder, A.S.; Meisner, C.A.; Sarkar, M.A.; Islam, M.S.; Sayre, K.D.; Duxbury, J.M.; Lauren, J.G. Effect of water management, arsenic and phosphorus levels on rice in a high-arsenic soil-water system: II. Arsenic uptake. Ecotoxicol. Environ. Saf. 2012, 80, 145–151. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Carey, M.; Meharg, A.A. Inorganic arsenic removal in rice bran by percolating cooking water. Food Chem. 2017, 234, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Gray, P.J.; Conklin, S.D.; Todorov, T.I.; Kasko, S.M. Cooking rice in excess water reduces both arsenic and enriched vitamins in the cooked grain. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 78–85. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Kalt, W.; Ryan, D.A.J.; Duy, J.C.; Prior, R.; Ehlenfeldt, M.K.; Vander Kloet, S.P. Interspecific variation in anthocyanins, phenolics, and antioxidant capacity among genotypes of highbush and lowbush blueberries (Vaccinium section cyanococcus spp.). J. Agric. Food Chem. 2001, 49, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Howard, L.R.; Brownmille, C.R.; Prior, R.L. Influence of extrusion processing on procyanidin composition and total anthocyanin contents of blueberry pomace. J. Food Sci. 2009, 74, H52–H58. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Mai Le, P.; et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef]
- Patras, N.; Brunton, C.; O’Donnell, B. Effect of thermal processing on anthocyanin stability in foods: Mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Grace, M.H.; Kuhn, P.; Cheng, D.M.; Plundrich, N.; Poulev, A.; Howell, A.; Fridlender, B.; Lila, M.A.; Raskin, I. Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chem. 2012, 131, 1193–1200. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.P.; Jin, J.; Huang, L.Y.; Ye, J.H.; Liang, Y.R. Using defatted rice bran as a bioadsorbent for carrying tea catechins. J. Food Sci. 2015, 80, C2134–C2139. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Kuhn, P.; Poulev, A.; Oren, A.; Lila, M.A.; Fridlender, B.; Raskin, I. Biochemical analysis and in vivo hypoglycemic activity of a grape polyphenol-soybean flour complex. J. Agric. Food Chem. 2012, 60, 8860–8865. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Kuhn, P.; Krueger, C.G.; Moskal, K.; Lila, M.A.; Raskin, I. Concord grape pomace polyphenols complexed to soy protein isolate are stable and hypoglycemic in diabetic mice. J. Agric. Food Chem. 2013, 61, 11428–11433. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Guzman, I.; Roopchand, D.E.; Moskal, K.; Cheng, D.M.; Pogrebnyak, N.; Raskin, I.; Howell, A.; Lila, M.A. Stable binding of alternative protein-enriched food matrices with concentrated cranberry bioflavonoids for functional food applications. J. Agric. Food Chem. 2013, 61, 6856–6864. [Google Scholar] [CrossRef] [PubMed]
- Guzman, I.; Grace, M.H.; Yousef, G.G.; Raskin, I.; Lila, M.A. Novel strategies for capturing health-protective mango phytochemicals in shelf stable food matrices. Int. J. Food Sci. Nutr. 2015, 66, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Chaney, R.L.; Green, C.E.; Lehotay, S.J. Inter-laboratory validation of an inexpensive streamlined method to measure inorganic arsenic in rice grain. Anal. Bioanal. Chem. 2018, 410, 5703–5710. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Wrolstad, R.E. Extraction of anthocyanins and polyphenolics from blueberry processing waste. J. Food Sci. 2004, 69, C564–C573. [Google Scholar] [CrossRef]
- Nicoué, E.E.; Savard, S.; Belkacemi, K. Anthocyanins in wild blueberries of Quebec: Extraction and identification. J. Agric. Food Chem. 2007, 55, 5626–5635. [Google Scholar] [CrossRef]
- Mei, X.; Qin, H.; Wang, J.; Wang, G.; Liu, C.; Cai, Y. Studies on physicochemical characteristics of anthocyanin from super dark maize. J. Food Nutr. Res. 2014, 2, 109–114. [Google Scholar] [CrossRef]
- Rawel, H.M.; Meidtner, K.; Kroll, J. Binding of selected phenolic compounds to proteins. J. Agric. Food Chem. 2013, 53, 4228–4235. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.; Cisneros-Zevallos, L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, D.; Czapski, J. Colour changes of a preparation from red cabbage during storage in a model system. Food Chem. 2007, 104, 709–714. [Google Scholar] [CrossRef]
- Reyes, L.F.; Cisneros-Zevallos, L. Degradation kinetics and colour of anthocyanins in aqueous extracts of purple- and red-flesh potatoes (Solanum tuberosum L.). Food Chem. 2007, 100, 885–894. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Y.H.; Cao, Y.; Zhu, Y.; Rathinasabapathi, B.; Ma, L.Q. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front. Plant Sci. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; Matsumoto, E.; Shindo, K.; Nishimura, T. Effects of polishing, cooking, and storing on total arsenic and arsenic species concentrations in rice cultivated in Japan. Food Chem. 2015, 168, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Raab, A.; Baskaran, C.; Feldmann, J.; Meharg, A.A. Cooking rice in a high water to rice ratio reduces inorganic arsenic content. J. Environ. Monit. 2009, 11, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Heitkemper, D.T.; Vela, N.P.; Stewart, K.R.; Westphal, C.S. Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry. J. Anal. Atom Spectrom. 2001, 16, 299–306. [Google Scholar] [CrossRef]
- Gogoi, P.; Adhikari, P.; Maji, T.K. Bioremediation of arsenic from water with citric acid cross-linked water hyacinth (E. crassipes) root powder. Environ. Monit. Assess. 2017, 189, 383. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Nath, B.; Sarkar, S.; Islam, S.M.; Bundschuh, J.; Chatterjee, D.; Hidalgo, M. Application of natural citric acid sources and their role on arsenic removal from drinking water: A green chemistry approach. J. Hazard. Mater. 2013, 262, 1167–1175. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme. 37th Session. In Proceedings of the Distribution of the Report of the Eighth Session of the Codex Committee on Contaminants in Foods, Geneva, Switzerland, 14–18 July 2014; Available online: http://www.fao.org/ (accessed on 1 September 2014).



| Glucose (mg/g) | Fructose (mg/g) | Sucrose (mg/g) | Total (mg/g) | |
|---|---|---|---|---|
| Blueberry Juice (Dried) | 359 ± 1.2 f | 355 ± 1.7 f | 0.61 ± 0.07 | 715 ± 0.64 f |
| Brown Flour + Juice | 81.3 ± 0.49 d | 81.5 ± 0.49 d | N/D | 163 ± 0.71 d |
| Defatted Bran + Juice | 113 ± 0.24 e | 113 ± 1.2 e | N/D | 227 ± 1.0 e |
| Pomace | 65.4 ± 2.9 c | 65.2 ± 3.4 c | N/D | 131 ± 6.3 c |
| Brown Flour + Pomace Extract | 7.81 ± 0.21 a | 7.48 ± 0.28 a | N/D | 15.3 ± 0.49 a |
| Defatted Bran + Pomace Extract | 14.5 ± 0.53 b | 14.1 ± 0.24 b | N/D | 28.6 ± 0.28 b |
| Rice–Blueberry Enrichment | Days | Lightness (L*) | Chroma (C*) | Hue Angle (h) | Color Difference (ΔE) |
|---|---|---|---|---|---|
| Brown Flour + Juice | 0 | 30.9 ± 0.1 a | 10.3 ± 0.1 a | 13.4 ± 0.4 d | 13.3 ± 0.8 b |
| 105 | 43.9 ± 0.8 de | 13.4 ± 0.4 d | 9.01 ± 0.4 d | ||
| Brown Flour + Pomace Extract | 0 | 32.7 ± 0.9 b | 11.8 ± 0.3 c | 356.9 ± 0.152 f | 11.1 ± 0.2 a |
| 105 | 43.2 ± 0.1 cd | 15.5 ± 0.4 f | 357.8 ± 0.072 g | ||
| Defatted Bran + Juice | 0 | 30.5 ± 0.6 a | 10.6 ± 0.1 a | 1.82 ± 0.1 a | 14.7 ± 0.4 c |
| 105 | 44.6 ± 0.5 f | 14.0 ± 0.1 e | 14.1 ± 0.2 e | ||
| Defatted Bran + Pomace Extract | 0 | 33.0 ± 0.9 b | 11.2 ± 0.4 b | 358.3 ± 0.3 h | 11.5 ± 0.4 a |
| 105 | 42.7 ± 0.5 c | 17.5 ± 0.1 g | 2.6 ± 0.09 b |
| Inorganic As (ppm) | Total As (ppm) | |
|---|---|---|
| Rice Bran | 0.610 ± 0.015 c | 0.847 ± 0.021 c |
| Rice Bran + Juice | 0.485 ± 0.017 b | 0.668 ± 0.018 b |
| Rice Bran + Pomace Extract | 0.295 ± 0.019 a | 0.445 ± 0.011 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boue, S.M.; Daigle, K.; Beaulieu, J.C.; Heiman, M. Rice Flour and Bran Enriched with Blueberry Polyphenols Increases Storage Stability and Decreases Arsenic Content in Bran. Foods 2019, 8, 276. https://doi.org/10.3390/foods8070276
Boue SM, Daigle K, Beaulieu JC, Heiman M. Rice Flour and Bran Enriched with Blueberry Polyphenols Increases Storage Stability and Decreases Arsenic Content in Bran. Foods. 2019; 8(7):276. https://doi.org/10.3390/foods8070276
Chicago/Turabian StyleBoue, Stephen M., Kim Daigle, John C. Beaulieu, and Mark Heiman. 2019. "Rice Flour and Bran Enriched with Blueberry Polyphenols Increases Storage Stability and Decreases Arsenic Content in Bran" Foods 8, no. 7: 276. https://doi.org/10.3390/foods8070276