Total, Neutral, and Polar Lipids of Brewing Ingredients, By-Products and Beer: Evaluation of Antithrombotic Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
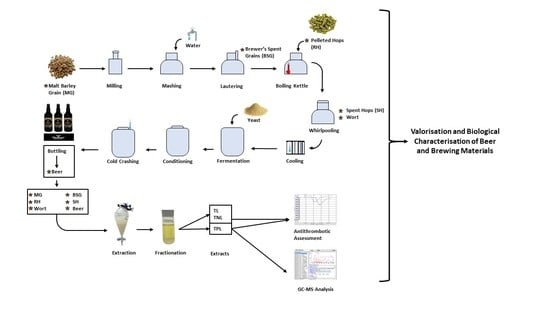
2.2. Beer Production and Samples Assessed
2.3. Extraction and Isolation of the TL, TNL, and TPL Extracts
2.4. Platelet Aggregation Assay
2.5. Gas Chromatography-Mass Spectrometry
2.6. Statistical Analysis
3. Results
3.1. Lipid Extraction and Fractionation of Beer, and Brewing Materials
3.2. Gas Chromatography-Mass Spectrometry Analysis
3.3. Platelet Aggregation Assay Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Schwarzinger, M.; Thiébaut, S.P.; Baillot, S.; Mallet, V.; Rehm, J. Alcohol use disorders and associated chronic disease—A national retrospective cohort study from france. BMC Public Health 2017, 18, 43. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. The potential role of dietary platelet-activating factor inhibitors in cancer prevention and treatment. Adv. Nutr. 2019, 10, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.C.; do Rio, R.F.; Lollo, P.C.B.; Ferreira, I.M.P.L.V.O. Moderate alcoholic beer consumption: The effects on the lipid profile and insulin sensitivity of adult men. J. Food Sci. 2017, 82, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759. [Google Scholar] [CrossRef]
- De Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef]
- Lombardi, G.; Vannini, S.; Blasi, F.; Marcotullio, M.C.; Dominici, L.; Villarini, M.; Cossignani, L.; Moretti, M. In vitro safety/protection assessment of resveratrol and pterostilbene in a human hepatoma cell line (HepG2). Nat. Prod. Commun. 2015, 10, 1403–1408. [Google Scholar] [CrossRef]
- Lordan, R.; Nasopoulou, C.; Tsoupras, A.; Zabetakis, I. The anti-inflammatory properties of food polar lipids. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–34. [Google Scholar]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef]
- Jaberi, N.; Soleimani, A.; Pashirzad, M.; Abdeahad, H.; Mohammadi, F.; Khoshakhlagh, M.; Khazaei, M.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Role of thrombin in the pathogenesis of atherosclerosis. J. Cell. Biochem. 2019, 120, 4757–4765. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [Green Version]
- Tsopanoglou, N.E.; Maragoudakis, M.E. Thrombin’s central role in angiogenesis and pathophysiological processes. Eur. Cytokine Netw. 2009, 20, 171–179. [Google Scholar]
- Jennings, L.K. Mechanisms of platelet activation: Need for new strategies to protect against platelet-mediated atherothrombosis. Thromb. Haemost. 2009, 101, 248–257. [Google Scholar] [CrossRef]
- Lordan, R.; O’Keeffe, E.; Dowling, D.; Mullally, M.; Heffernan, H.; Tsoupras, A.; Zabetakis, I. The in vitro antithrombotic properties of ale, lager, and stout beers. Food Biosci. 2019, 28, 83–88. [Google Scholar] [CrossRef]
- Seefeldt, H.F.; Larsen, F.H.; Viereck, N.; Petersen, M.A.; Engelsen, S.B. Lipid composition and deposition during grain filling in intact barley (hordeum vulgare) mutant grains as studied by 1H HR MAS NMR. J. Cereal Sci. 2011, 54, 442–449. [Google Scholar] [CrossRef]
- Shewry, P.R. Chapter 7-minor components of the barley grain: Minerals, lipids, terpenoids, phenolics, and vitamins. In Barley, 2nd ed.; Shewry, P.R., Ullrich, S.E., Eds.; AACC International Press: St. Paul, MI, USA, 2014; pp. 169–192. [Google Scholar]
- Cozzolino, D.; Degner, S. An overview on the role of lipids and fatty acids in barley grain and their products during beer brewing. Food Res. Int. 2016, 81, 114–121. [Google Scholar] [CrossRef]
- Marion, D.; Dubreil, L.; Douliez, J.-P. Functionality of lipids and lipid-protein interactions in cereal-derived food products. OCL 2003, 10, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The chemical profiling of fatty acids during the brewing process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef]
- Bamforth, C.W. Progress in brewing science and beer production. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 161–176. [Google Scholar] [CrossRef]
- Moonjai, N.; Verstrepen, K.J.; Shen, H.Y.; Derdelinckx, G.; Verachtert, H.; Delvaux, F.R. Linoleic acid supplementation of a cropped brewing lager strain: Effects on subsequent fermentation performance with serial repitching. J. I. Brew. 2003, 109, 262–272. [Google Scholar] [CrossRef]
- Moonjai, N.; Verstrepen, K.J.; Delvaux, F.R.; Derdelinckx, G.; Verachtert, H. The effects of linoleic acid supplementation of cropped yeast on its subsequent fermentation performance and acetate ester synthesis. J. Inst. Brew. 2002, 108, 227–235. [Google Scholar] [CrossRef]
- Gordon, R.; Power, A.; Chapman, J.; Chandra, S.; Cozzolino, D. A review on the source of lipids and their interactions during beer fermentation that affect beer quality. Fermentation 2018, 4, 89. [Google Scholar] [CrossRef]
- Olajire, A.A. The brewing industry and environmental challenges. J. Clean. Prod. 2012. [Google Scholar] [CrossRef]
- Mussatto, S.; Dragone, G.; Roberto, I. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Connolly, A.; Piggott, C.O.; FitzGerald, R.J. Characterisation of protein-rich isolates and antioxidative phenolic extracts from pale and black brewers’ spent grain. Int. J. Food Sci. Technol. 2013, 48, 1670–1681. [Google Scholar] [CrossRef]
- Niemi, P.; Tamminen, T.; Smeds, A.; Viljanen, K.; Ohra-aho, T.; Holopainen-Mantila, U.; Faulds, C.B.; Poutanen, K.; Buchert, J. Characterization of lipids and lignans in brewer’s spent grain and its enzymatically extracted fraction. J. Agric. Food Chem. 2012, 60, 9910–9917. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Mathias, T.R.; de Mello, P.P.M.; ervulo, E.F.C. Solid wastes in brewing process: A review. J. Brew. Distill. 2014, 5, 1–9. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Zabetakis, I.; Lordan, R. Platelet aggregometry assay for evaluating the effects of platelet agonists and antiplatelet compounds on platelet function in vitro. MethodsX 2019, 6, 63–70. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. In vitro antithrombotic properties of salmon (salmo salar) phospholipids in a novel food-grade extract. Mar. Drugs 2019, 17, 62. [Google Scholar] [CrossRef]
- Bravi, E.; Marconi, O.; Sileoni, V.; Perretti, G. Determination of free fatty acids in beer. Food Chem. 2017, 215, 341–346. [Google Scholar] [CrossRef]
- Bravi, E.; Benedetti, P.; Marconi, O.; Perretti, G. Determination of free fatty acids in beer wort. Food Chem. 2014, 151, 374–378. [Google Scholar] [CrossRef]
- Bravi, E.; Marconi, O.; Perretti, G.; Fantozzi, P. Influence of barley variety and malting process on lipid content of malt. Food Chem. 2012, 135, 1112–1117. [Google Scholar] [CrossRef]
- Evans, D.E.; Goldsmith, M.; Redd, K.S.; Nischwitz, R.; Lentini, A. Impact of mashing conditions on extract, its fermentability, and the levels of wort free amino nitrogen (fan), β-glucan, and lipids. J. Am. Soc. Brew. Chem. 2012, 70, 39–49. [Google Scholar] [CrossRef]
- Michiu, D.; Socaci, S.A.; Jimborean, M.A.; Mudura, E.; Fărcaş, A.C.; Biriş-Dorhoi, S.E.; Tofană, M. Determination of volatile markers from magnum hops in beer by in-tube extraction—gas chromatography-mass spectrometry. Anal. Lett. 2018, 51, 2967–2980. [Google Scholar] [CrossRef]
- Praet, T.; Van Opstaele, F.; De Causmaecker, B.; Aerts, G.; De Cooman, L. Heat-induced changes in the composition of varietal hop essential oils via wort boiling on a laboratory scale. J. Am. Soc. Brew. Chem. 2016, 74, 212–223. [Google Scholar] [CrossRef]
- Yoon, M.-Y.; Cha, B.; Kim, J.-C. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus lupulus l., a very popular beer ingredient and medicinal plant: Overview of its phytochemistry, its bioactivity, and its biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Eyres, G.; Dufour, J.-P. 22-hop essential oil: Analysis, chemical composition and odor characteristics. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 239–254. [Google Scholar]
- Anness, B.J. Lipids of barley, malt and adjuncts. J. I. Brew. 1984, 90, 315–318. [Google Scholar] [CrossRef]
- Blasi, F.; Montesano, D.; Simonetti, M.S.; Cossignani, L. A simple and rapid extraction method to evaluate the fatty acid composition and nutritional value of goji berry lipid. Food Anal. Method. 2017, 10, 970–979. [Google Scholar] [CrossRef]
- Morrison, W.R.; Coventry, A.M. Extraction of lipids from cereal starches with hot aqueous alcohols. Starch Stärke 1985, 37, 83–87. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. I. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Aromadendrene oxide 2, induces apoptosis in skin epidermoid cancer cells through ROS mediated mitochondrial pathway. Life Sci. 2018, 197, 19–29. [Google Scholar] [CrossRef]
- Kennedy, G.L. Toxicity of adipic acid. Drug Chem. Toxicol. 2002, 25, 191–202. [Google Scholar] [CrossRef]
- Lentz, M. The impact of simple phenolic compounds on beer aroma and flavor. Fermentation 2018, 4, 20. [Google Scholar] [CrossRef]
- Shinohara, T.; Kubodera, S.; Yanagida, F. Distribution of phenolic yeasts and production of phenolic off-flavors in wine fermentation. J. Biosci. Bioeng. 2000, 90, 90–97. [Google Scholar] [CrossRef]
- Varsha, K.K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K.M. 2,4-di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated lactococcus sp. Int. J. Food Microbiol. 2015, 211, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, J.K.; Kim, H.K.; Harris, K.; Kim, C.-J.; Park, G.G.; Park, C.-S.; Shin, D.-H. 2,4-di-tert-butylphenol from sweet potato protects against oxidative stress in pc12 cells and in mice. J. Med. Food 2013, 16, 977–983. [Google Scholar] [CrossRef]
- Bijak, M.; Ziewiecki, R.; Saluk, J.; Ponczek, M.; Pawlaczyk, I.; Krotkiewski, H.; Wachowicz, B.; Nowak, P. Thrombin inhibitory activity of some polyphenolic compounds. Med. Chem. Res. 2014, 23, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Policegoudra, R.S.; Goswami, S.; Aradhya, S.M.; Chatterjee, S.; Datta, S.; Sivaswamy, R.; Chattopadhyay, P.; Singh, L. Bioactive constituents of homalomena aromatica essential oil and its antifungal activity against dermatophytes and yeasts. J. Mycol. Med. 2012, 22, 83–87. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. Β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Tognolini, M.; Barocelli, E.; Ballabeni, V.; Bruni, R.; Bianchi, A.; Chiavarini, M.; Impicciatore, M. Comparative screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006, 78, 1419–1432. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Karantonis, H.C.; Andriotis, M.; Demopoulos, C.A.; Zabetakis, I. Antibacterial and anti-PAF activity of lipid extracts from sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata). Food Chem. 2008, 111, 433–438. [Google Scholar] [CrossRef]
- Sioriki, E.; Smith, T.K.; Demopoulos, C.A.; Zabetakis, I. Structure and cardioprotective activities of polar lipids of olive pomace, olive pomace-enriched fish feed and olive pomace fed gilthead sea bream (Sparus aurata). Food Res. Int. 2016, 83, 143–151. [Google Scholar] [CrossRef]
- Lordan, R.; Walsh, A.M.; Crispie, F.; Finnegan, L.; Cotter, P.D.; Zabetakis, I. The effect of ovine milk fermentation on the antithrombotic properties of polar lipids. J. Funct. Foods 2019, 54, 289–300. [Google Scholar] [CrossRef]
- Demopoulos, C.A.; Karantonis, H.C.; Antonopoulou, S. Platelet-activating factor—A molecular link between atherosclerosis theories. Eur. J. Lipid Sci. Technol. 2003, 105, 705–716. [Google Scholar] [CrossRef]
- Mori, T.A.; Beilin, L.J.; Burke, V.; Morris, J.; Ritchie, J. Interactions between dietary fat, fish, and fish oils and their effects on platelet function in men at risk of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 279–286. [Google Scholar] [CrossRef]
- Yoshida, K.; Nagatoishi, S.; Kuroda, D.; Suzuki, N.; Murata, T.; Tsumoto, K. Phospholipid membrane fluidity alters ligand binding activity of a g protein-coupled receptor by shifting the conformational equilibrium. Biochemistry 2019, 58, 504–508. [Google Scholar] [CrossRef]

| Sample | TL (g/100 g) | TNL (mg/100 g) | TNL (% TL) | TPL (g/100 g) | TPL (% TL) |
|---|---|---|---|---|---|
| MG | 0.70 ± 0.10 a | 100 ± 30 a | 13.6 ± 2.9 c | 0.52 ± 0.05 a | 74.5 ± 3.5 b |
| BSG | 1.05 ± 0.19 a | 550 ± 110 b | 52.6 ± 2.5 e | 0.41 ± 0.09 a | 38.9 ± 2.3 a |
| PH | 14.17 ± 2.18 b | 1630 ± 310 c | 11.5 ± 3.7 bc | 11.60 ± 1.68 b | 79.4 ± 8.6 bc |
| SH | 0.75 ± 0.06 a | 160 ± 10 a | 21.4 ± 3.0 d | 0.55 ± 0.07 a | 72.6 ± 4.1 b |
| Wort | 0.03 ± 0.00 a | 2.0 ± 1.0 a | 5.4 ± 1.6 ab | 0.03 ± 0.00 a | 84.5 ± 8.9 bc |
| Beer | 0.02 ± 0.00 a | 0.3 ± 0.1 a | 1.7 ± 0.4 a | 0.02 ± 0.01 a | 91.3 ± 2.7 c |
| Fatty Acids | Malt Grain | Spent Grain | Pelleted Hops | Spent Hops | Wort | Beer | |
|---|---|---|---|---|---|---|---|
| 8:0 | Caprylic acid | ND | ND | 0.04 ± 0.01 b | 0.02 ± 0.01 ab | 0.01 ± 0.00 a | ND |
| 10:0 | Capric acid | ND | ND | 0.06 ± 0.00 b | ND | 0.01 ± 0.00 a | 0.02 ± 0.01 a |
| 12:0 | Lauric acid | 0.17 ± 0.02 d | ND | 0.02 ± 0.00 a | 0.03 ± 0.01 a | 0.09 ± 0.01 c | 0.07 ± 0.00 b |
| 12:1 | cis-Lauroleic acid | ND | ND | 0.05 ± 0.01 | ND | ND | ND |
| 14:0 | Myristic acid | 0.62 ± 0.06 b | 0.69 ± 0.06 b | 0.33 ± 0.05 a | 1.04 ± 0.11 c | 1.58 ± 0.04 d | 1.55 ± 0.09 d |
| 14:1 | cis-Myristoleic acid | ND | 0.41 ± 0.11 | ND | ND | ND | ND |
| 15:0 | Pentadecylic acid | 0.31 ± 0.04 a | 0.22 ± 0.07 a | 0.49 ± 0.14 b | 0.25 ± 0.01 a | 0.17 ± 0.02 a | 0.16 ± 0.02 a |
| 16:0 | Palmitic acid | 19.83 ± 0.93 a | 27.86 ± 0.80 b | 22.05 ± 1.23 a | 30.38 ± 1.0 c | 31.80 ± 0.60 c | 32.34 ± 0.68 c |
| 16:1 | cis-Palmitoleic acid | 0.39 ± 0.04 a | 0.30 ± 0.12 a | 2.35 ± 0.26 c | 1.15 ± 0.15 b | 0.60 ± 0.02 a | 0.33 ± 0.21 a |
| 17:0 | Margaric acid | 0.18 ± 0.02 a | ND | 1.24 ± 0.03 d | 0.46 ± 0.03 c | 0.24 ± 0.02 b | 0.23 ± 0.01 ab |
| 17:1 | cis-Heptadecenoic acid | 0.09 ± 0.01 a | ND | 0.75 ± 0.04 d | 0.36 ± 0.03 c | 0.20 ± 0.01 b | 0.20 ± 0.03 b |
| 18:0 | Stearic acid | 2.63 ± 0.62 ab | 2.23 ± 0.25 a | 2.85 ± 0.08 abc | 3.85 ± 0.18 d | 3.61 ± 0.09 cd | 3.32 ± 0.10 bcd |
| 18:1 c9 | cis-Oleic acid | 9.04 ± 0.19 d | 8.81 ± 0.44 d | 4.39 ± 0.11 a | 6.67 ± 0.26 d | 6.12 ± 0.24 bc | 5.62 ± 0.18 b |
| 18:1 t13 | trans-Oleic acid | 0.66 ± 0.02 a | 1.01 ± 0.09 ab | 1.27 ± 0.16 bc | 1.82 ± 0.24 d | 1.42 ± 0.09 c | 1.26 ± 0.14 bc |
| 18:2 c9, c12 | Linoleic acid | 56.67 ± 0.77 e | 51.83 ± 1.59 d | 25.46 ± 1.5 a | 40.68 ± 0.34 b | 44.78 ± 0.06 c | 43.48 ± 1.55 bc |
| 18:3 c6, c9, c12 | γ-Linolenic acid | ND | ND | 0.58 ± 0.02 b | 0.12 ± 0.01 a | ND | ND |
| 18:3 c9, c12, c15 | α-Linolenic acid | 6.80 ± 0.95 abc | 5.87 ± 0.54 ab | 23.42 ± 1.4 d | 8.83 ± 0.92 c | 7.72 ± 0.71 bc | 5.13 ± 0.26 a |
| 20:0 | Arachidic acid | 0.58 ± 0.10 ab | 0.78 ± 0.17 bc | 1.02 ± 0.04 c | 0.48 ± 0.07 a | ND | ND |
| 20:1 c13 | Eicosenoic acid | ND | ND | 0.25 ± 0.05 a | 0.54 ± 0.04 c | 0.39 ± 0.02 b | ND |
| 20:2 c11, c14 | Eicosadienoic acid | ND | ND | 1.00 ± 0.05 c | 0.49 ± 0.07 b | 0.27 ± 0.01 a | 0.31 ± 0.04 a |
| 20:4 c5, c8, c11, c14 | Arachidonic acid | ND | ND | ND | ND | 0.52 ± 0.06 | 4.93 ± 0.02 |
| 20:5 c5, c8, c11, c14, c17 | Eicosapentaenoic acid | ND | ND | 0.53 ± 0.01 b | 0.16 ± 0.03 a | 0.41 ± 0.10 b | ND |
| 22:0 | Behenic acid | 0.30 ± 0.04 a | ND | 1.32 ± 0.32 c | 0.72 ± 0.13 b | 0.41 ± 0.02 ab | 0.29 ± 0.07 a |
| 22:1 | Erucic acid | 0.32 ± 0.09 a | ND | 0.41 ± 0.06 a | 0.34 ± 0.09 a | ND | ND |
| 22:6 c4, c7, c10, c13, c16, c19 | Docosahexaenoic acid | 0.47 ± 0.09 a | ND | 1.46 ± 0.17 b | 0.43 ± 0.13 a | ND | ND |
| ΣSFA | 24.43 ± 0.70 a | 31.79 ± 0.94 b | 29.40 ± 1.32 b | 37.23 ± 0.91 c | 37.94 ± 0.60 c | 37.97 ± 0.54c | |
| ΣMUFA | 10.67 ± 0.14 c | 10.53 ± 0.41 c | 9.47 ± 0.48 b | 10.86 ± 0.37 c | 9.13 ± 0.21 b | 7.42 ± 0.45 a | |
| ΣPUFA | 63.95 ± 1.53 c | 57.69 ± 1.09 b | 52.87 ± 2.66 a | 50.71 ± 0.67 a | 53.69 ± 0.60 a | 53.75 ± 1.76 a | |
| Volatiles | |||||||
| Hexanedioic acid | ND | ND | ND | ND | 0.28 ± 0.04 | 1.12 ± 0.21 | |
| Aromadendrene oxide | ND | ND | 1.77 ± 0.39 | 0.11 ± 0.02 | ND | ND | |
| 2,4-Di-tert-butylphenol | ND | ND | ND | ND | ND | 0.12 ± 0.01 | |
| β-Caryophyllene | ND | ND | 2.02 ± 0.37 b | 0.37 ± 0.05 a | ND | 0.07 ± 0.01 a | |
| 2-Dodecanone | ND | ND | 0.07 ± 0.05 | 0.04 ± 0.01 | ND | ND | |
| Cubenol | ND | ND | 0.24 ± 0.18 | ND | ND | ND | |
| Tau-Cadinol | ND | ND | 0.14 ± 0.08 | ND | ND | ND | |
| Tau-Muurolol | ND | ND | 0.29 ± 0.02 | ND | ND | 0.08 ± 0.00 | |
| ΣVolatiles | ND | ND | 8.90 ± 0.32 b | 0.95 ± 0.32 a | 0.28 ± 0.04 | 1.37 ± 0.22 a |
| Sample | TL | TNL | TPL |
|---|---|---|---|
| MG | 495 ± 105 b | 298 ± 89 a | 191 ± 58 ab |
| BSG | 69 ± 33 a | 610 ± 136 b | 617 ± 184 c |
| PH | 453 ± 109 b | 1088 ± 172 c | 473 ± 280 c |
| SH | 519 ± 81 b | 924 ± 166 c | 436 ± 142 bc |
| Wort | 70 ± 29 a | 175 ± 61 a | 58 ± 11 a |
| Beer | 6.4 ± 4.5 a | 248 ± 66 a | 7.8 ± 3.9 a |
| Sample | TL | TNL | TPL |
|---|---|---|---|
| MG | 112 ± 21 b | 433 ± 77 b | 247 ± 39 b |
| BSG | 87 ± 10 b | 409 ± 30 b | 203 ± 49 b |
| PH | 221 ± 42 c | 478 ± 97 b | 207 ± 51 b |
| SH | 155 ± 56 bc | 572 ± 76 b | 396 ± 62 c |
| Wort | 10 ± 3.7 a | 165 ± 61 a | 24 ± 17 a |
| Beer | 2.4 ± 0.9 a | 206 ± 73 a | 4.3 ± 3.0 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lordan, R.; O’Keeffe, E.; Tsoupras, A.; Zabetakis, I. Total, Neutral, and Polar Lipids of Brewing Ingredients, By-Products and Beer: Evaluation of Antithrombotic Activities. Foods 2019, 8, 171. https://doi.org/10.3390/foods8050171
Lordan R, O’Keeffe E, Tsoupras A, Zabetakis I. Total, Neutral, and Polar Lipids of Brewing Ingredients, By-Products and Beer: Evaluation of Antithrombotic Activities. Foods. 2019; 8(5):171. https://doi.org/10.3390/foods8050171
Chicago/Turabian StyleLordan, Ronan, Eoin O’Keeffe, Alexandros Tsoupras, and Ioannis Zabetakis. 2019. "Total, Neutral, and Polar Lipids of Brewing Ingredients, By-Products and Beer: Evaluation of Antithrombotic Activities" Foods 8, no. 5: 171. https://doi.org/10.3390/foods8050171