Quali-Quantitative Profile of Native Carotenoids in Kumquat from Brazil by HPLC-DAD-APCI/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
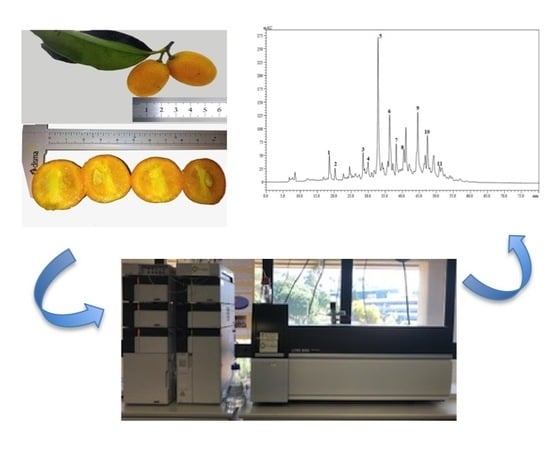
2.2. Collection and Preparation of the Samples
2.3. Moisture Analysis
2.4. Extraction of Carotenoids
2.5. Analysis of Carotenoids by HPLC-DAD-APCI-MS
2.6. Identification and Quantification of Carotenoids
3. Results and Discussion
3.1. Carotenoids Qualitative Profile of Brazilian Kumquat
3.2. Carotenoids Quantitative Profile of Brazilian Kumquat
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares; Ministério da Saúde: Rio de Janeiro, Brasília, 2009.
- The Plant List. Available online: http://www.theplantlist.org/tpl/record/kew-2724150 (accessed on 15 May 2019).
- Ogawa, K.; Kawasaki, A.; Omura, M.; Yoshida, T.; Ikoma, Y.; Yano, M. 3′,5′-Di-C-β-glucopyranosylphloretin, a flavonoid characteristic of the genus Fortunella. Phytochemistry 2001, 57, 737–742. [Google Scholar] [CrossRef]
- Pompeu Junior, J. Rootstocks and scions in the citriculture of the São Paulo State; In Proceedings of International Congress of Citrus Nurserymen, Ribeirão Preto, Brasil, 2001; pp. 75–82. [Google Scholar]
- Watanabe, H.S.; De Oliveira, S.L. Comercialização de frutas exóticas. Revista Brasileira de Fruticultura 2014, 36, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Kumquat (Fortunella japonica Swingle) juice: Flavonoid distribution and antioxidant properties. Food. Res. Int. 2011, 44, 2190–2197. [Google Scholar] [CrossRef]
- Koller, O.L. Citricultura Catarinense; Epagri: Florianópolis, Brazil, 2013.
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of flavonoid constituents in citrus fruits. J. Agric. Food Chem. 1999, 47, 3565–3571. [Google Scholar] [CrossRef]
- Schirra, M.; Palma, A.; D’Aquino, S.; Angioni, A.; Minello, E.V.; Melis, M.; Cabras, P. Influence of postharvest hot water treatment on nutritional and functional properties of kumquat (Fortunella japonica Lour. Swingle Cv. Ovale) fruit. J. Agric. Food Chem. 2007, 56, 455–460. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Liu, Y.; Liu, H.; Shang, Y. Evaluating effects of ellagic acid on the quality of kumquat fruits during storage. Sci. Hortic. 2018, 227, 244–254. [Google Scholar] [CrossRef]
- Lou, S.N.; Ho, C.T. Phenolic compounds and biological activities of small-size citrus: Kumquat and calamondin. J. Food Drug Anal. 2017, 25, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.N.; Lai, Y.C.; Hsu, Y.S.; Ho, C.T. Phenolic content, antioxidant activity and effective compounds of kumquat extracted by different solvents. Food Chem. 2016, 197, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.N.; Lai, Y.C.; Huang, J.D.; Ho, C.T.; Ferng, L.H.A.; Chang, Y.C. Drying effect on flavonoid composition and antioxidant activity of immature kumquat. Food Chem. 2015, 171, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Yang, K.M.; Huang, T.C.; Wu, M.L. Traditional small-size citrus from Taiwan: Essential oils, bioactive compounds and antioxidant capacity. Medicines 2017, 4, 28. [Google Scholar] [CrossRef]
- Salvo, A.; Bruno, M.; La Torre, G.L.; Vadalà, R.; Mottese, A.F.; Saija, E.; Mangano, V.; Casale, K.E.; Cicero, N.; Dugo, G. Interdonato lemon from Nizza di Sicilia (Italy): Chemical composition of hexane extract of lemon peel and histochemical investigation. Nat. Prod. Res. 2016, 30, 1517–1525. [Google Scholar] [CrossRef]
- Salvo, A.; Costa, R.; Albergamo, A.; Arrigo, S.; Rotondo, A.; La Torre, G.L.; Mangano, V.; Dugo, G. An in-depth study of the volatile variability of chinotto (Citrus myrtifolia Raf.) induced by the extraction procedure. Eur. Food Res. Technol. 2019. [Google Scholar] [CrossRef]
- Costa, R.; Salvo, A.; Rotondo, A.; Bartolomeo, G.; Pellizzeri, V.; Saija, E.; Arrigo, S.; Interdonato, M.; Trozzi, A.; Dugo, G. Combination of separation and spectroscopic analytical techniques: Application to compositional analysis of a minor citrus species. Nat. Prod. Res. 2018, 32, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- Agócs, A.; Nagy, V.; Szabó, Z.; Márk, L.; Ohmacht, R.; Deli, J. Comparative study on the carotenoid composition of the peel and the pulp of different citrus species. Innov. Food. Sci. Emerg. Technol. 2007, 8, 390–394. [Google Scholar] [CrossRef]
- Huyskens, S.; Timberg, R.; Gross, J. Pigment and plastid ultrastructural changes in Kumquat (Fortunella margarita) «Nagami» during ripening. J. Plant. Physiol. 1985, 118, 61–72. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chuang, Y.C.; Ku, Y.H. Quantitation of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chem. 2007, 102, 1163–1171. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Johnson, E.J.; Krinsky, N.I. Carotenoids and coronary heart disease. In Carotenoids; Springer: Basel, Switzerland, 2009; pp. 287–300. [Google Scholar]
- Giuffrida, D.; Dugo, P.; Salvo, A.; Saitta, M.; Dugo, G. Free carotenoid and carotenoid ester composition in native orange juices of different varieties. Fruits 2010, 65, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Huang, X.; Lv, S.; Pan, S. Carotenoid profiling of red navel orange “Cara Cara” harvested from five regions in China. Food Chem. 2017, 232, 788–798. [Google Scholar] [CrossRef]
- Donadio, L.C. Dicionário das Frutas; UNESP: Jaboticabal, Brazil, 2007. [Google Scholar]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [Green Version]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2010. [Google Scholar]
- Rodriguez-Amaya, D. Food Carotenoids: Chemistry, Biology and Technology; John Wiley & Sons: Hoboken, NZ, USA, 2015. [Google Scholar]
- Amorim-Carrilho, K.; Cepeda, A.; Fente, C.; Regal, P. Review of methods for analysis of carotenoids. Trends. Analyt. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Fu, H.; Xie, B.; Fan, G.; Ma, S.; Zhu, X.; Pan, S. Effect of esterification with fatty acid of β-cryptoxanthin on its thermal stability and antioxidant activity by chemiluminescence method. Food Chem. 2010, 122, 602–609. [Google Scholar] [CrossRef]
- Bunea, A.; Socaciu, C.; Pintea, A. Xanthophyll esters in fruits and vegetables. Not. Bot. Horti Agrobot. Cluj Napoca 2014, 42, 310. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Iida, K.; Madono, Y.; Yungyuen, W.; Yahata, M.; Yamawaki, K.; Kato, M. Identification and quantitative analysis of β-cryptoxanthin and β-citraurin esters in Satsuma mandarin fruit during the ripening process. Food Chem. 2017, 234, 356–364. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Matsuta, A.; Matsutani, K.; Yamawaki, K.; Yahata, M.; Wahyudi, A.; Motohashi, R.; Kato, M. Enzymatic formation of β-citraurin from β-cryptoxanthin and zeaxanthin by carotenoid cleavage dioxygenase4 in the flavedo of citrus fruit. Plant Physiol. 2013, 163, 682–695. [Google Scholar] [CrossRef]
- Murillo, E.; Giuffrida, D.; Menchaca, D.; Dugo, P.; Torre, G.; Meléndez-Martinez, A.J.; Mondello, L. Native carotenoids composition of some tropical fruits. Food Chem. 2013, 140, 825–836. [Google Scholar] [CrossRef]
- Iskandar, A.R.; Liu, C.; Smith, D.E.; Hu, K.Q.; Choi, S.W.; Ausman, L.M.; Wang, X.D. β-Cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev. Res. 2012, 6, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, K.; Morimoto, S.I.; Shirakura, Y.; Mukai, K.; Sugiyama, T.; Tokuji, Y.; Ohnishi, M. Mechanism of visceral fat reduction in Tsumura Suzuki obese, diabetes (TSOD) mice orally administered β-cryptoxanthin from Satsuma mandarin oranges (Citrus unshiu Marc). J. Agric. Food Chem. 2011, 59, 12342–12351. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of carotenoid β-cryptoxanthin in bone homeostasis. J. Biomed. Sci. 2012, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, D.E.; Weller, P.; Wolters, M.; Hahn, A. Plasma response to a single dose of dietary β-cryptoxanthin esters from papaya (Carica papaya L.) or non-esterified β-cryptoxanthin in adult human subjects: A comparative study. Br. J. Nutr. 2003, 90, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Matsubara, A.; Uchikata, T.; Iwasaki, Y.; Morimoto, S.; Kan, K.; Ookura, T.; Fukusaki, E.; Bamba, T. Investigation of β-cryptoxanthin fatty acid ester compositions in citrus fruits Cultivated in Japan. Food Nutr. Sci. 2013, 4, 98. [Google Scholar]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Mariutti, L.R.B. Carotenoid esters in foods-A review and practical directions on analysis and occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Khachik, F. Carotenoids in food. In Carotenoids; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Springer: Basel, Switzerland, 2009. [Google Scholar]




| Compound | Identification | Rt (min) | PDA (λnm) | MS (APCI-) m/z |
|---|---|---|---|---|
| 1 | Lutein | 18.5 | 445, 473 | 568 |
| 2 | Zeaxanthin | 20.2 | 449, 476 | 568 |
| 3 | β-cryptoxanthin | 28.4 | 428, 451, 478 | 552 |
| 4 | β-citraurin-caproate | 29.9 | 454 | 586 |
| 5 | β-citraurin-laurate | 32.8 | 455 | 614 |
| 6 | β-citraurin-myristate | 36.2 | 453 | 642 |
| 7 | β-carotene | 38.1 | 426, 451, 476 | 536 |
| 8 | β-citraurin-palmitate | 40.4 | 455 | 642 |
| 9 | β-cryptoxanthin-laurate | 44.5 | 428, 450, 478 | 734 |
| 10 | β-cryptoxanthin-myristate | 47.3 | 428, 450, 477 | 762 |
| 11 | β-cryptoxanthin-palmitate | 50.9 | 428, 451, 478 | 790 |
| Compound | Carotenoid | Content in Lyophilized Kumquat (µg/100 g) * | Content in Fresh Kumquat (µg/100 g) * | Carotenoid Composition (%) |
|---|---|---|---|---|
| 1 | Lutein | 873.05 ± 93.51 | 144.35 ± 15.45 | 6.61 |
| 2 | Zeaxanthin | 469.55 ± 25.52 | 77.63 ± 4.21 | 3.55 |
| 3 | β-Cryptoxanthin | 634.93 ± 55.91 | 104.98 ± 9.25 | 4.80 |
| 4 | β-Citraurin-caproate | 721.81 ± 20.04 | 119.34 ± 3.32 | 5.46 |
| 5 | β-Citraurin-laurate | 3673.28 ± 81.78 | 607.33 ± 13.63 | 27.80 |
| 6 | β-Citraurin-myristate | 421.88 ± 9.52 | 69.75 ± 1.58 | 3.20 |
| 7 | β-Carotene | 726.96 ± 110.60 | 120.19 ± 18.29 | 5.50 |
| 8 | β-Citraurin-palmitate | 801.27 ± 66.35 | 132.48 ± 10.98 | 6.06 |
| 9 | β-Cryptoxanthin-laurate | 3342.25 ± 126.10 | 552.59 ± 20.75 | 25.31 |
| 10 | β-Cryptoxanthin-myristate | 927.77 ± 82.92 | 153.39 ± 13.71 | 7.02 |
| 11 | β-Cryptoxanthin-palmitate | 623.73 ± 46.34 | 103.13 ± 7.67 | 4.72 |
| Total carotenes | 726.96 | 120.19 | 5.50 | |
| Total free xanthophylls | 1977.53 | 326.96 | 14.96 | |
| Total esterified xanthophylls | 10511.99 | 1738.01 | 79.54 | |
| Total carotenoids | 13,216.48 | 2185.16 | 100 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro-Sant’Ana, H.M.; Anunciação, P.C.; Souza, C.S.e.; de Paula Filho, G.X.; Salvo, A.; Dugo, G.; Giuffrida, D. Quali-Quantitative Profile of Native Carotenoids in Kumquat from Brazil by HPLC-DAD-APCI/MS. Foods 2019, 8, 166. https://doi.org/10.3390/foods8050166
Pinheiro-Sant’Ana HM, Anunciação PC, Souza CSe, de Paula Filho GX, Salvo A, Dugo G, Giuffrida D. Quali-Quantitative Profile of Native Carotenoids in Kumquat from Brazil by HPLC-DAD-APCI/MS. Foods. 2019; 8(5):166. https://doi.org/10.3390/foods8050166
Chicago/Turabian StylePinheiro-Sant’Ana, Helena Maria, Pamella Cristine Anunciação, Clarice Silva e Souza, Galdino Xavier de Paula Filho, Andrea Salvo, Giacomo Dugo, and Daniele Giuffrida. 2019. "Quali-Quantitative Profile of Native Carotenoids in Kumquat from Brazil by HPLC-DAD-APCI/MS" Foods 8, no. 5: 166. https://doi.org/10.3390/foods8050166