Effect of Hydrocolloid Gums on the Pasting, Thermal, Rheological and Textural Properties of Chickpea Starch
Abstract
:1. Introduction
2. Results and Discussions
Pasting Properties of Starch Gum Blends
3. Dynamic Rheology
4. Flow Behavior and Temperature Dependency
5. Texture Profile Analysis of Starch Gum Blends Gel
6. Freeze–Thaw Stability of Starch Gum Blends Gel
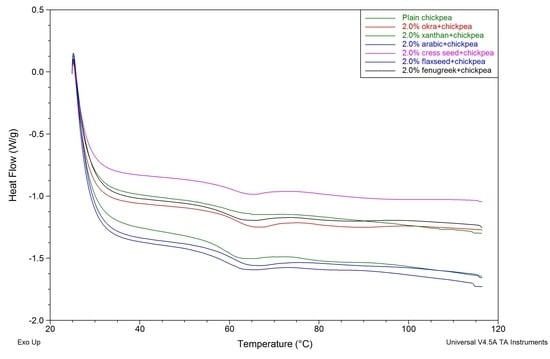
7. Differential Scanning Calorimetry
8. Conclusions
9. Material and Methods
9.1. Materials
9.2. Methods
Isolation of Chickpea Starch
9.3. Extraction of Gums
Garden Cress Seed Gum
9.4. Fenugreek Gum
9.5. Flaxseed Gum
9.6. Okra Gum
9.7. Preparation of Starch Gum Blends
9.8. Pasting Properties of Starch Gum Blends
9.9. Dynamic Rheology, Steady Flow Behavior and Temperature Dependency
9.10. Texture Profile Analysis of Starch Gum Blends Gel
9.11. Freeze–Thaw Stability of Starch Gum Blends Gel
9.12. Differential Scanning Calorimetry
10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sciarini, L.; Maldonado, F.; Ribotta, P.; Perez, G.; Leon, A. Chemical composition and functional properties of Gleditsia triacanthos gum. Food Hydrocoll. 2009, 23, 306–313. [Google Scholar] [CrossRef]
- Rosell, C.; Rojas, J.; De Barber, C.B. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Gallagher, E. 11 Functionality of Starches and Hydrocolloids in Gluten-Free Foods. In Gluten-Free Food Science and Technology; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hüttner, E.; Arendt, E. Recent advances in gluten-free baking and the current status of oats. Trends Food Sci. Technol. 2010, 21, 303–312. [Google Scholar] [CrossRef]
- Yang, X.; Feng, M.Q.; Sun, J.; Xu, X.L.; Zhou, G.H. The influence of flaxseed gum on the retrogradation of maize starch. Int. J. Food Sci. Technol. 2017, 52, 2654–2660. [Google Scholar] [CrossRef]
- Shrivastava, M.; Yadav, R.B.; Yadav, B.S.; Dangi, N. Effect of incorporation of hydrocolloids on the physicochemical, pasting and rheological properties of colocasia starch. J. Food Meas. Charact. 2018, 12, 1177–1185. [Google Scholar] [CrossRef]
- Santana, Á.L.; Meireles, M.A.A. New starches are the trend for industry applications: A review. Food Public Health 2014, 4, 229–241. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Chavan, J.; Kadam, S.; Salunkhe, D.; Beuchat, L.R. Biochemistry and technology of chickpea (Cicer arietinum L.) seeds. Crit. Rev. Food Sci. Nutr. 1987, 25, 107–158. [Google Scholar] [CrossRef]
- Miao, M.; Zhang, T.; Jiang, B. Characterisations of kabuli and desi chickpea starches cultivated in China. Food Chem. 2009, 113, 1025–1032. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, B.; Li, M.-N.; Xie, Y.; Chen, H.-Q. Effects of glutenin and gliadin modified by protein-glutaminase on pasting, rheological properties and microstructure of potato starch. Food Chem. 2018, 253, 148–155. [Google Scholar] [CrossRef]
- Raina, C.; Singh, S.; Bawa, A.; Saxena, D. Some characteristics of acetylated, cross-linked and dual modified Indian rice starches. Eur. Food Res. Technol. 2006, 223, 561–570. [Google Scholar] [CrossRef]
- Pongsawatmanit, R.; Temsiripong, T.; Ikeda, S.; Nishinari, K. Influence of tamarind seed xyloglucan on rheological properties and thermal stability of tapioca starch. J. Food Eng. 2006, 77, 41–50. [Google Scholar] [CrossRef]
- Temsiripong, T.; Pongsawatmanit, R.; Ikeda, S.; Nishinari, K. Influence of xyloglucan on gelatinization and retrogradation of tapioca starch. Food Hydrocoll. 2005, 19, 1054–1063. [Google Scholar] [CrossRef]
- Correa, M.; Ferrero, C.; Puppo, C.; Brites, C. Rheological properties of rice–locust bean gum gels from different rice varieties. Food Hydrocoll. 2013, 31, 383–391. [Google Scholar] [CrossRef]
- Inglett, G.E.; Chen, D.; Lee, S. Rheological properties of barley and flaxseed composites. Food Nutr. Sci. 2013, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Alamri, M.S.; Mohamed, A.; Hussain, S.; Xu, J. Effect of Okra extract on properties of wheat, corn and rice starches. J. Foodagric. Environ. 2012, 10, 217–222. [Google Scholar]
- Lee, M.; Baek, M.; Cha, D.; Park, H.; Lim, S. Freeze–thaw stabilization of sweet potato starch gel by polysaccharide gums. Food Hydrocoll. 2002, 16, 345–352. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, J.; Singh, H.; McCarthy, O.J. Starch–cassia gum interactions: A microstructure–Rheology study. Food Chem. 2008, 111, 1–10. [Google Scholar] [CrossRef]
- Alamri, M.S.; Mohamed, A.A.; Hussain, S. Effect of okra gum on the pasting, thermal, and viscous properties of rice and sorghum starches. Carbohydr. Polym. 2012, 89, 199–207. [Google Scholar] [CrossRef]
- Alamri, M.S. Sweet potato/potato starch and Abelmoschus esculentus-gum blends: Thermal and textural properties. Starch Stärke 2014, 66, 132–141. [Google Scholar] [CrossRef]
- Yadav, K.; Yadav, B.S.; Yadav, R.B.; Dangi, N. Physicochemical, pasting and rheological properties of colocasia starch as influenced by the addition of guar gum and xanthan gum. J. Food Meas. Charact. 2018, 12, 2666–2676. [Google Scholar] [CrossRef]
- Alamri, M.S.; Mohamed, A.A.; Hussain, S. Effects of alkaline-soluble okra gum on rheological and thermal properties of systems with wheat or corn starch. Food Hydrocoll. 2013, 30, 541–551. [Google Scholar] [CrossRef]
- Jane, J.l.; Chen, Y.; Lee, L.; McPherson, A.; Wong, K.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- Leite, T.D.; Nicoleti, J.F.; Penna, A.L.B.; Franco, C.M.L. Effect of addition of different hydrocolloids on pasting, thermal, and rheological properties of cassava starch. Food Sci. Technol. 2012, 32, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.H.; Cui, S.W. Steady and dynamic shear rheological properties of extrusion modified fenugreek gum solutions. Food Sci. Biotechnol. 2011, 20, 1663–1668. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.-J.; Li, D.; Özkan, N.; Chen, X.D.; Mao, Z.-H. Effect of flaxseed gum addition on rheological properties of native maize starch. J. Food Eng. 2008, 89, 87–92. [Google Scholar] [CrossRef]
- Alloncle, M.; Doublier, J.-L. Viscoelastic properties of maize starch/hydrocolloid pastes and gels. Food Hydrocoll. 1991, 5, 455–467. [Google Scholar] [CrossRef]
- Lee, Y.; Chang, Y.H. Effects of galactomannan addition on rheological, pasting and physical properties of water chestnut starch. J. Texture Stud. 2015, 46, 58–66. [Google Scholar] [CrossRef]
- Singh, A.; Geveke, D.J.; Yadav, M.P. Improvement of rheological, thermal and functional properties of tapioca starch by using gum arabic. LWT-Food Sci. Technol. 2017, 80, 155–162. [Google Scholar] [CrossRef]
- Ji, N.; Qiu, C.; Xu, Y.; Xiong, L.; Sun, Q. Differences in rheological behavior between normal and waxy corn starches modified by dry heating with hydrocolloids. Starch Stärke 2017, 69, 1600332. [Google Scholar] [CrossRef]
- Warner, D.D.; Araujo, O.E. Kinetics of rheological properties of acacia solutions. J. Pharm. Sci. 1971, 60, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W.; Chang, Y.H. Steady and dynamic shear rheological properties of buckwheat starch-galactomannan mixtures. Prev. Nutr. Food Sci. 2012, 17, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaari, N.; Sulaiman, R.; Cheok, C. Rheological properties of native and modified corn starches in the presence of hydrocolloids. Int. Food Res. J. 2017, 24, 2082–2089. [Google Scholar]
- Choi, H.M.; Yoo, B. Rheology of mixed systems of sweet potato starch and galactomannans. Starch Stärke 2008, 60, 263–269. [Google Scholar] [CrossRef]
- Urlacher, B.; Noble, O. Xanthan gum. In Thickening and Gelling Agents for Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 284–311. [Google Scholar]
- Krystyjan, M.; Sikora, M.; Adamczyk, G.; Tomasik, P. Caramel sauces thickened with combinations of potato starch and xanthan gum. J. Food Eng. 2012, 112, 22–28. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.-J.; Li, D.; Özkan, N.; Li, S.-J.; Mao, Z.-H. Rheological properties of waxy maize starch and xanthan gum mixtures in the presence of sucrose. Carbohydr. Polym. 2009, 77, 472–481. [Google Scholar] [CrossRef]
- Sharoba, A.; Senge, B.; El-Mansy, H.; Bahlol, H.E.; Blochwitz, R. Chemical, sensory and rheological properties of some commercial German and Egyptian tomato ketchups. Eur. Food Res. Technol. 2005, 220, 142–151. [Google Scholar] [CrossRef]
- Kim, C.; Yoo, B. Rheological properties of rice starch–xanthan gum mixtures. J. Food Eng. 2006, 75, 120–128. [Google Scholar] [CrossRef]
- Marcotte, M.; Hoshahili, A.R.T.; Ramaswamy, H. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res. Int. 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Arocas, A.; Sanz, T.; Fiszman, S. Improving effect of xanthan and locust bean gums on the freeze-thaw stability of white sauces made with different native starches. Food Hydrocoll. 2009, 23, 2478–2484. [Google Scholar] [CrossRef]
- Varela, M.S.; Navarro, A.S.; Yamul, D.K. Effect of hydrocolloids on the properties of wheat/potato starch mixtures. Starch Stärke 2016, 68, 753–761. [Google Scholar] [CrossRef]
- Brennan, C.S.; Suter, M.; Matia-Merino, L.; Luethi, T.; Ravindran, G.; Goh, K.; Ovortrup, J. Gel and pasting behaviour of fenugreek-wheat starch and fenugreek–wheat flour combinations. Starch Stärke 2006, 58, 527–535. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.S.; Chen, H.H.; Li, Q.Q. Effect of sodium alginate on the gelatinization and retrogradation properties of two tuber starches. Cereal Chem. 2018, 95, 445–455. [Google Scholar] [CrossRef]
- Feng, M.; Yang, X.; Sun, J.; Xu, X.; Zhou, G. Study on retrogradation of maize starch–flaxseed gum mixture under various storage temperatures. Int. J. Food Sci. Technol. 2018, 53, 1287–1293. [Google Scholar] [CrossRef]
- Tang, M.; Hong, Y.; Gu, Z.; Zhang, Y.; Cai, X. The effect of xanthan on short and long-term retrogradation of rice starch. Starch Stärke 2013, 65, 702–708. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.-S.; Chen, H.-H.; Li, Q.-Q.; Wang, Q. The gelatinization and retrogradation properties of wheat starch with the addition of stearic acid and sodium alginate. Food Hydrocoll. 2018, 81, 77–86. [Google Scholar] [CrossRef]
- Yamazaki, E.; Sago, T.; Kasubuchi, Y.; Imamura, K.; Matsuoka, T.; Kurita, O.; Nambu, H.; Matsumura, Y. Improvement on the freeze–thaw stability of corn starch gel by the polysaccharide from leaves of Corchorus olitorius L. Carbohydr. Polym. 2013, 94, 555–560. [Google Scholar] [CrossRef]
- Weber, F.H.; Queiroz, F.P.C.; Chang, Y.K. Freeze-thaw stability of normal, waxy and high amylose corn starch gels with added guar and xanthan gums. Food Sci. Technol. 2008, 28, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Alamri, M.; Al-Ruquie, I.; Hussain, S.; Mohamed, A.; Mahmood, K. Effect of potassium phosphate on the thermal, pasting, and flowing properties of chickpea and potato starches. Qual. Assur. Saf. Crop. Foods 2015, 7, 431–440. [Google Scholar] [CrossRef]
- Karazhiyan, H.; Razavi, S.M.; Phillips, G.O. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. 2011, 25, 915–920. [Google Scholar] [CrossRef]
- Shahzad, S.A.; Hussain, S.; Alamri, M.S.; Mohamed, A.A.; Ahmed, A.S.; Ibraheem, M.A.; Qasem, A.; Akram, A. Use of Hydrocolloid Gums to Modify the Pasting, Thermal, Rheological, and Textural Properties of Sweet Potato Starch. Int. J. Polym. Sci. 2019, 2019, 6308591. [Google Scholar] [CrossRef]
- Qian, K.; Cui, S.; Wu, Y.; Goff, H. Flaxseed gum from flaxseed hulls: Extraction, fractionation, and characterization. Food Hydrocoll. 2012, 28, 275–283. [Google Scholar] [CrossRef]
- Alamri, M.; Hussain, S.; Mohamed, A.; Qasem, A.; Mahmood, K. Effect of sodium phosphate on the pasting, thermal, and rheological properties of potato and chickpea starches. Qual. Assur. Saf. Crop. Foods 2016, 8, 249–259. [Google Scholar] [CrossRef]



| Parameter | Gum % | Control | Arabic | Xanthan | Cress Seed | Fenugreek | Flaxseed | Okra |
|---|---|---|---|---|---|---|---|---|
| Peak Viscosity (cP) a | 0.5 | 3807 ± 22 d | 3496 ± 14 f | 4256 ± 16 a | 3928 ± 21 c | 4040 ± 79 b | 3703 ± 33 e | 3370 ± 03 g |
| 2.0 | 3807 ± 22 c | 2804 ± 16 g | 5611 ± 109 a | 3257 ± 14 e | 4311 ± 95 b | 3140 ± 15 f | 3528 ± 37 d | |
| Breakdown Viscosity (cP) | 0.5 | 1350 ± 47 a | 1153 ± 44 b,c | 1069 ± 31 c | 1354 ± 79 a | 1270 ± 118 a,b | 1211 ± 40 b | 1157 ± 14 b,c |
| 2.0 | 1350 ± 47 a | 0789 ± 22 c | 1419 ± 09 a | 1085 ± 11 b | 1333 ± 51 a | 1035 ± 11 b | 1073 ± 116 b | |
| Final Viscosity (cP) | 0.5 | 6146 ± 109 b,c | 5688 ± 38 d | 6317 ± 102 a | 6067 ± 22 c | 6207 ± 72 a,b | 5620 ± 43 d | 5115 ± 14 e |
| 2.0 | 6146 ± 109 c | 4958 ± 55 d | 6760 ± 190 a | 5039 ± 39 d | 6330 ± 51 b | 4757 ± 07 e | 4935 ± 17 d | |
| Setback (cP) | 0.5 | 3691 ± 133 a | 3344 ± 94 b,c | 3140 ± 120 c | 3503 ± 68 a,b | 3417 ± 269 b | 3146 ± 32 c | 2899 ± 05 d |
| 2.0 | 3691 ± 133 a | 2943 ± 19 c | 2550 ± 78 d,e | 2867 ± 33 c | 3351 ± 37 b | 2671 ± 24 d | 2461 ± 87 e | |
| Pasting Temperature (°C) | 0.5 | 69.83 ± 0.4 b | 70.61 ± 0.4 a | 70.56 ± 0.4 a | 70.68 ± 0.4 a | 70.56 ± 0.5 a | 71.03 ± 0.1 a | 71.16 ± 0.1 a |
| 2.0 | 69.83 ± 0.4 d | 70.61 ± 0.4 c,d | 68.11 ± 1.0 e | 72.10 ± 0.4 b | 71.73 ± 0.2 b | 71.30 ± 0.4 b,c | 73.45 ± 0.1 a |
| Parameters | Calculated Setback Value Due to Starch Replacement | RVA Measured Values | Difference a Due to Gum | % Reduction b in Setback Due to Gum | ||||
|---|---|---|---|---|---|---|---|---|
| Gum % | 0.5 | 2.0 | 0.5 | 2.0 | 0.5 | 2.0 | 0.5 | 2.0 |
| Control | 3691 | 3691 | 3691 | 3691 | − | − | − | − |
| Arabic | 3672 | 3617 | 3344 | 2943 | 328 | 674 | 9 | 19 |
| Xanthan | 3672 | 3617 | 3140 | 2550 | 532 | 1067 | 14 | 30 |
| Cress seed | 3672 | 3617 | 3503 | 2867 | 169 | 750 | 05 | 21 |
| Fenugreek | 3672 | 3617 | 3417 | 3351 | 255 | 321 | 07 | 07 |
| Flaxseed | 3672 | 3617 | 3146 | 2671 | 526 | 946 | 14 | 26 |
| Okra | 3672 | 3617 | 2899 | 2461 | 773 | 1156 | 21 | 32 |
| Gum % | K′ | n′ | R2 | K″ | n″ | R2 | |
|---|---|---|---|---|---|---|---|
| Control | − | 2.600 b | 0.101 b | 0.72 a | 1.209 c | 0.277 c | 0.89 a |
| Arabic | 0.5 | 2.498 c | 0.106 b | 0.70 a | 1.043 d | 0.274 c | 0.87 b |
| Xanthan | 0.5 | 2.613 b | 0.140 a | 0.73 a | 1.222 c | 0.320 a | 0.91 a |
| Cress seed | 0.5 | 2.682 b | 0.114 b | 0.63 b | 1.319 b | 0.248 d | 0.82 c |
| Fenugreek | 0.5 | 2.641 b | 0.111 b | 0.64 b | 1.212 c | 0.292 b | 0.92 a |
| Flaxseed | 0.5 | 2.543 c | 0.111 b | 0.64 b | 1.085 d | 0.278 c | 0.86 b |
| Okra | 0.5 | 2.857 a | 0.115 b | 0.54 c | 1.469 a | 0.271 c | 0.71 d |
| Control | − | 2.600 a | 0.101 b | 0.72 b | 1.209 b | 0.277 b | 0.89 b |
| Arabic | 2.0 | 2.428 c | 0.103 d | 0.70 b,c | 0.943 d | 0.266 b | 0.77 d |
| Xanthan | 2.0 | 2.424 c | 0.136 a | 0.79 a | 1.559 a | 0.199 d | 0.89 b |
| Cress seed | 2.0 | 2.548 b | 0.109 d | 0.68 c | 1.114 c | 0.254 b,c | 0.88 b |
| Fenugreek | 2.0 | 2.405 c | 0.108 d | 0.68 c | 0.954 d | 0.312 a | 0.92 a |
| Flaxseed | 2.0 | 2.556 b | 0.132 a | 0.63 d | 1.059 d | 0.319 a | 0.84 c |
| Okra | 2.0 | 2.598 a | 0.118 d | 0.60 e | 1.239 b | 0.239 c | 0.87 b |
| Temperature | Parameter | Gum % | Control | Arabic | Xanthan | Cress Seed | Fenugreek | Flaxseed | Okra |
|---|---|---|---|---|---|---|---|---|---|
| 25 °C | 1K | 0.5 | 5.86 d | 6.07 c | 7.56 a | 7.09 b | 5.83 d | 5.98 c,d | 6.14 c |
| 2.0 | 5.86 e | 6.56 c | 10.7 a | 6.26 d | 5.88 e | 6.22 d | 7.07 b | ||
| 2n | 0.5 | 0.40 a | 0.36 b | 0.34 c | 0.35 b,c | 0.39 a | 0.39 a | 0.36 b | |
| 2.0 | 0.40 a | 0.35 c | 0.32 d | 0.36 b,c | 0.36 b,c | 0.37 b | 0.34 c,d | ||
| R2 | 0.5 | 0.99 a | 0.99 a | 0.99 a | 0.98 a | 0.99 a | 0.99 a | 0.99 a | |
| 2.0 | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.98 a | ||
| 45 °C | K | 0.5 | 5.40 c | 4.90 e | 6.32 a | 5.78 b | 5.29 d | 5.40 c | 5.27 d |
| 2.0 | 5.40 c | 5.19 c | 9.01 a | 6.04 b | 4.26 d | 5.04 c | 6.16 b | ||
| n | 0.5 | 0.40 b | 0.42 a | 0.38 c | 0.36 d | 0.39 b,c | 0.40 b | 0.36 d | |
| 2.0 | 0.40 a | 0.41 a | 0.34 d | 0.38 b | 0.40 a | 0.40 a | 0.36 c | ||
| R2 | 0.5 | 0.99 a | 0.98 a | 0.99 a | 0.98 a | 0.99 a | 0.99 a | 0.98 a | |
| 2.0 | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a | ||
| 65 °C | K | 0.5 | 2.90 b | 2.77 d,e | 3.79 a | 2.92 b | 2.72 e | 2.80 c | 2.64 f |
| 2.0 | 2.90 c,d | 2.72 e | 7.80 a | 2.99 c | 2.84 d | 2.84 d | 3.43 b | ||
| n | 0.5 | 0.48 b | 0.48 b | 0.46 c | 0.46 c | 0.49 a | 0.49 a | 0.47 c | |
| 2.0 | 0.48 a | 0.49 a | 0.36 d | 0.46 b | 0.46 b | 0.48 a | 0.43 c | ||
| R2 | 0.5 | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a | |
| 2.0 | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a | 0.99 a |
| Parameters | µo (Pa sn) A | Ea (J/mol K−1) B | R2 | |||
|---|---|---|---|---|---|---|
| Gum % | 0.5 | 2.0 | 0.5 | 2.0 | 0.5 | 2.0 |
| Control | 1.04 × 10−4 e | 1.04 × 10−4 e | 14,485 d | 14,485 d | 0.80 | 0.80 |
| Arabic | 2.02 × 10−5 d | 3.93 × 10−6 c | 16,262 b,c | 18,243 a | 0.91 | 0.91 |
| Xanthan | 2.04 × 10−4 d | 4.95 × 10−1 b | 14,305 d | 6628 e | 0.91 | 0.99 |
| Cress seed | 5.74 × 10−7 b | 6.69 × 10−5 a | 20,576 a | 15,171 c | 0.87 | 0.76 |
| Fenugreek | 3.33 × 10−5 c | 4.59 × 10−5 b | 15,706 c | 15,194 c | 0.82 | 0.99 |
| Flaxseed | 3.75 × 10−5 c | 2.17 × 10−5 d | 15,637 c | 16,247 b | 0.82 | 0.91 |
| Okra | 7.52 × 10−6 a | 1.01 × 10−4 e | 17,428 b | 14,941 d | 0.86 | 0.87 |
| Parameters | Gum % | Control | Arabic | Xanthan | Cress Seed | Fenugreek | Flaxseed | Okra |
|---|---|---|---|---|---|---|---|---|
| Hardness (N) | 0.5 | 8.23 ± 0.21 b | 8.31 ± 0.18 b | 7.47 ± 0.14 d | 8.29 ± 0.06 b | 8.58 ± 0.12 a | 7.93 ± 0.19 c | 7.82 ± 0.09 c |
| 2.0 | 8.23 ± 0.21 b | 8.17 ± 0.12 b,c | 6.63 ± 0.15 d | 8.02 ± 0.13 b,c | 8.77 ± 0.09 a | 7.99 ± 0.19 b,c | 7.90 ± 0.17 c | |
| Cohesiveness | 0.5 | 0.53 ± 0.02 b | 0.51 ± 0.01 b | 0.56 ± 0.02 a | 0.53 ± 0.01 b | 0.47 ± 0.02 c | 0.56 ± 0.02 a | 0.57 ± 0.01 a |
| 2.0 | 0.53 ± 0.02 d,e | 0.51 ± 0.01 e | 0.61 ± 0.01 a | 0.54 ± 0.01 c,d | 0.45 ± 0.02 f | 0.57 ± 0.01 b | 0.55 ± 0.01 b,c | |
| Springiness (mm) | 0.5 | 9.90 ± 0.00 b,c | 9.80 ± 0.00 c,d | 10.17 ± 0.06 a | 9.80 ± 0.10 c,d | 9.73 ± 0.06 d | 9.97 ± 0.06 b | 9.97 ± 0.15 b |
| 2.0 | 9.90 ± 0.00 c | 10.07 ± 0.06 b | 10.27 ± 0.06 a | 9.87 ± 0.06 c | 9.63 ± 0.06 d | 10.0 ± 0.17 b,c | 9.93 ± 0.06 b,c | |
| Adhesiveness (mJ) | 0.5 | 0.03 ± 0.06 c | 0.13 ± 0.06 a,b | 0.17 ± 0.06 a,b | 0.17 ± 0.06 a,b | 0.10 ± 0.00 b,c | 0.17 ± 0.06 a,b | 0.20 ± 0.00 a |
| 2.0 | 0.03 ± 0.06 c | 0.23 ± 0.06 a | 0.13 ± 0.06 a,b | 0.17 ± 0.06 a,b | 0.10 ± 0.00 b,c | 0.13 ± 0.06 a,b | 0.17 ± 0.06 a,b | |
| Gumminess N | 0.5 | 4.34 ± 0.16 a,b | 4.21 ± 0.11 b,c | 4.21 ± 0.08 b,c | 4.40 ± 0.07 a,b | 4.03 ± 0.14 c | 4.47 ± 0.03 a | 4.45 ± 0.08 a |
| 2.0 | 4.34 ± 0.16 b | 4.19 ± 0.04 b,c | 4.04 ± 0.17 c | 4.33 ± 0.14 b | 3.98 ± 0.19 c | 4.55 ± 0.09 a | 4.37 ± 0.05 a,b | |
| Chewiness (N.mm) | 0.5 | 42.93 ± 1.56 a,b | 41.24 ± 1.06 b,c | 42.78 ± 1.02 a,b | 43.07 ± 0.86 a,b | 39.33 ± 1.46 c | 44.52 ± 0.17 a | 44.88 ± 1.45 a |
| 2.0 | 42.93 ± 1.56 b | 42.22 ± 0.63 b | 41.51 ± 0.92 b | 42.73 ± 1.59 b | 38.30 ± 2.05 c | 45.52 ± 0.18 a | 43.44 ± 0.57 a b |
| Days | 7th Day | 14th Day | 21st Day | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Gum % | 0.5 | 2.0 | 0.5 | 2.0 | 0.5 | 2.0 | 0.5 | 2.0 |
| Control | 2.72 ± 0.11 c,d | 2.72 ± 0.49 b | 1.66 ± 0.35 b | 1.66 ± 0.54 b | 1.51 ± 0.16 a | 1.51 ± 0.16 a | 5.90 ± 0.39 c | 5.90 ± 0.39 b |
| Arabic | 2.95 ± 0.73 b,c,d | 2.24 ± 0.23 c | 2.35 ± 0.45 a | 1.74 ± 0.18 b | 0.50 ± 0.26 c | 1.30 ± 0.22 a,b | 5.81 ± 0.09 c | 5.29 ± 0.19 c,d |
| Xanthan | 2.46 ± 0.62 d | 1.32 ± 0.38 d | 0.60 ± 0.11 c | 0.34 ± 0.11 c | 0.66 ± 0.30 c | 0.87 ± 0.09 c | 3.64 ± 0.23 e | 2.54 ± 0.34 e |
| Cress seed | 2.66 ± 0.30 c,d | 4.05 ± 0.24 a | 1.77 ± 0.05 b | 0.65 ± 0.28 c | 0.58 ± 0.21 c | 0.51 ± 0.20 d | 5.02 ± 0.12 d | 5.22 ± 0.61 c,d |
| Fenugreek | 3.60 ± 0.26 b | 2.79 ± 0.02 b | 1.63 ± 0.31 b | 1.66 ± 0.02 b | 1.43 ± 0.26 a | 0.49 ± 0.09 d | 6.67 ± 0.24 b | 4.94 ± 0.10 d |
| Flaxseed | 3.40 ± 0.32 b,c | 2.68 ± 0.13 b | 2.38 ± 0.11 a | 1.79 ± 0.39 b | 0.81 ± 0.18 b,c | 1.12 ± 0.28 b,c | 6.60 ± 0.08 b | 5.59 ± 0.17 b,c |
| Okra | 5.34 ± 0.37 a | 3.82 ± 0.30 a | 2.52 ± 0.34 a | 2.59 ± 0.58 a | 1.16 ± 0.21 a,b | 0.45 ± 0.12 d | 9.03 ± 0.35 a | 6.87 ± 0.26 a |
| Parameters | T0 (°C) A | Tp (°C) B | ΔH (J/g) C | |||
|---|---|---|---|---|---|---|
| Gum % | 0.5 | 2.0 | 0.5 | 2.0 | 0.5 | 2.0 |
| Control | 54.71 ± 0.28 a,b | 54.71 ± 0.28 d | 63.10 ± 0.32 c | 63.10 ± 0.32 d | 13.25 ± 0.54 b | 13.25 ± 0.54 b |
| Arabic | 55.10 ± 0.20 a,b | 55.71 ± 0.22 b | 63.33 ± 0.15 b,c | 63.64 ± 0.20 c | 11.99 ± 0.93 c | 11.70 ± 0.87 c |
| Xanthan | 55.19 ± 0.67 a,b | 55.44 ± 0.15 b,c | 63.59 ± 0.02 b,c | 64.41 ± 0.51 b | 04.24 ± 0.14 d | 04.00 ± 0.36 d |
| Cress seed | 54.60 ± 0.33 b | 55.03 ± 0.18 c,d | 63.83 ± 0.63 b | 64.32 ± 0.26 b | 13.91 ± 0.76 a,b | 14.74 ± 0.44 a |
| Fenugreek | 55.55 ± 0.62 a | 55.76 ± 0.40 b | 63.80 ± 0.17 b | 64.11 ± 0.24 b,c | 14.14 ± 0.05 a,b | 14.34 ± 0.18 a |
| Flaxseed | 54.96 ± 0.54 a,b | 55.48 ± 0.52 b,c | 63.58 ± 0.17 b,c | 64.02 ± 0.19 b,c | 14.63 ± 0.31 a | 14.81 ± 0.56 a |
| Okra | 54.89 ± 0.44 a,b | 56.51 ± 0.29 a | 64.53 ± 0.06 a | 65.58 ± 0.03 a | 13.42 ± 0.63 b | 13.19 ± 0.83 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzad, S.A.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Ibraheem, M.A.; Qasem, A.A.A. Effect of Hydrocolloid Gums on the Pasting, Thermal, Rheological and Textural Properties of Chickpea Starch. Foods 2019, 8, 687. https://doi.org/10.3390/foods8120687
Shahzad SA, Hussain S, Mohamed AA, Alamri MS, Ibraheem MA, Qasem AAA. Effect of Hydrocolloid Gums on the Pasting, Thermal, Rheological and Textural Properties of Chickpea Starch. Foods. 2019; 8(12):687. https://doi.org/10.3390/foods8120687
Chicago/Turabian StyleShahzad, Syed Ali, Shahzad Hussain, Abdellatif A. Mohamed, Mohamed S. Alamri, Mohamed A. Ibraheem, and Akram A. Abdo Qasem. 2019. "Effect of Hydrocolloid Gums on the Pasting, Thermal, Rheological and Textural Properties of Chickpea Starch" Foods 8, no. 12: 687. https://doi.org/10.3390/foods8120687