Inactivation of Escherichia Coli and Salmonella Using 365 and 395 nm High Intensity Pulsed Light Emitting Diodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
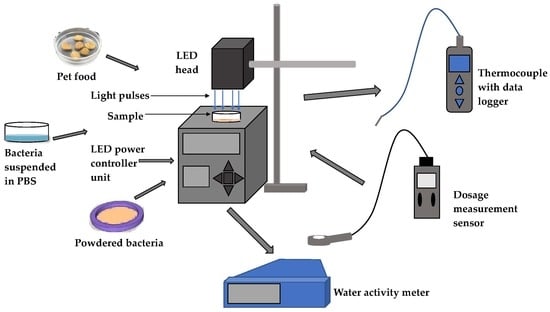
2.2. Light Emitting Diode (LED) System
2.3. Inactivation of E. coli and Salmonella enterica Cells in Phosphate Buffer Saline
2.4. Inactivation of Dry E. coli and Salmonella enterica
2.5. Inactivation of Salmonella Cells on Low-aw Pet Food Pellets
2.6. Weight Loss, Water Content and Water Sorption Isotherms of the Treated Samples
2.7. Statistical Analysis
3. Results
3.1. Antibacterial Efficacy of 365 and 395 nm LED Treatments
3.2. Effect of Power Levels on the Antibacterial Efficacy of the LEDs
3.3. Antibacterial Efficacy of LED in Low-aw Pet Foods
3.4. Change in Temperature during LED Treatment
3.5. Change in Water Content and Water Activity of Bacterial Samples and Pet Foods during LED Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cordier, J.-L. Methodological and sampling challenges to testing spices and low-water activity food for the presence of foodborne pathogens. In The Microbiological Safety of Low Water Activity Foods and Spices; Springer: New York, NY, USA, 2014; pp. 367–386. [Google Scholar]
- Carrasco, E.; Morales-Rueda, A.; García-Gimeno, R.M. Cross-contamination and recontamination by Salmonella in foods: A review. Food Res. Int. 2012, 45, 545–556. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Doyle, M.P.; Kornacki, J.L. The microbiological safety of spices and low-water activity foods: Correcting historic misassumptions. In The Microbiological Safety of Low Water Activity Foods and Spices; Springer: New York, NY, USA, 2014; pp. 3–13. [Google Scholar]
- Laroche, C.; Fine, F.; Gervais, P. Water activity affects heat resistance of microorganisms in food powders. Int. J. Food Microbiol. 2005, 97, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rojas, R.; Tang, J.; Wang, S.; Gao, M.; Kang, D.-H.; Mah, J.-H.; Gray, P.; Sosa-Morales, M.E.; López-Malo, A. Thermal inactivation of Salmonella Enteritidis PT 30 in almond kernels as influenced by water activity. J. Food Prot. 2013, 76, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.; Bingol, G.; Brandl, M.T.; Mchugh, T.H. Review of current technologies for reduction of Salmonella populations on almonds. Food Bioprocess Technol. 2012, 5, 2046–2057. [Google Scholar] [CrossRef]
- Chen, B.Y.; Lung, H.M.; Yang, B.B.; Wang, C.Y. Pulsed light sterilization of packaging materials. Food Packag. Shelf Life 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Elmnasser, N.; Guillou, S.; Leroi, F.; Orange, N.; Bakhrouf, A.; Federighi, M. Pulsed-light system as a novel food decontamination technology: A review. Can. J. Microbiol. 2007, 53, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, D.K.; Kang, D.H. Using UVC light-emitting diodes at wavelengths of 266 to 279 nanometers to inactivate foodborne pathogens and pasteurize sliced cheese. Appl. Environ. Microbiol. 2016, 82, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Kramer, B.; Wunderlich, J.; Muranyi, P. Pulsed light decontamination of endive salad and mung bean sprouts in water. Food Control 2017, 73, 367–371. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Lee, A.; Farber, J.M. Methods for the control of foodborne pathogens in low-moisture foods. Annu. Rev. Food Sci. Technol. 2018, 9, 177–208. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef]
- Kim, M.-J.; Miks-Krajnik, M.; Kumar, A.; Yuk, H.-G. Inactivation by 405 ± 5 nm light emitting diode on Escherichia coli O157:H7, Salmonella Typhimurium, and Shigella sonnei under refrigerated condition might be due to the loss of membrane integrity. Food Control 2016, 59, 99–107. [Google Scholar] [CrossRef]
- Li, G.Q.; Wang, W.L.; Huo, Z.Y.; Lu, Y.; Hu, H.Y. Comparison of UV-LED and low pressure UV for water disinfection: Photoreactivation and dark repair of Escherichia coli. Water Res. 2017, 126, 134–143. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, C.; Yuk, H.G.; Khoo, G.H.; Zhou, W. Application of light-emitting diodes in food production, postharvest preservation, and microbiological food safety. Compr. Rev. Food Sci. Food Saf. 2015, 14, 719–740. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Tadapaneni, R.K.; Xu, J.; Villa-Rojas, R.; Tang, J.; Carter, B.; Sablani, S.; Marks, B. Water activity change at elevated temperatures and thermal resistance of Salmonella in all purpose wheat flour and peanut butter. Food Res. Int. 2016, 81, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, A.; Syamaladevi, R.M.; Killinger, K.; Sablani, S.S. Ultraviolet-C light inactivation of Escherichia coli O157:H7 and Listeria monocytogenes on organic fruit surfaces. Int. J. Food Microbiol. 2015, 210, 136–142. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Lu, X.; Sablani, S.S.; Kumar Insan, S.; Adhikari, A.; Killinger, K.; Rasco, B.; Dhingra, A.; Bandyopadhyay, A.; Annapure, U.; et al. Inactivation of Escherichia coli population on fruit surfaces using ultraviolet-C light: Influence of fruit surface characteristics. Food Bioprocess Technol. 2013, 6, 2959–2973. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Lupien, S.L.; Bhunia, K.; Sablani, S.S.; Dugan, F.; Rasco, B.; Killinger, K.; Dhingra, A.; Ross, C. UV-C light inactivation kinetics of Penicillium expansum on pear surfaces: Influence on physicochemical and sensory quality during storage. Postharvest Biol. Technol. 2014, 87, 27–32. [Google Scholar] [CrossRef]
- de Gruijl, F.R. P53 mutations as a marker of skin cancer risk: Comparison of UVA and UVB effects. Exp. Derm. 2002, 11, 37–39. [Google Scholar] [CrossRef]
- Shirai, A.; Watanabe, T.; Matsuki, H. Inactivation of foodborne pathogenic and spoilage micro-organisms using ultraviolet-A light in combination with ferulic acid. Lett. Appl. Microbiol. 2017, 64, 96–102. [Google Scholar] [CrossRef]
- Hamamoto, A.; Mori, M.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Akutagawa, M.; Ikehara, T.; Nakaya, Y.; Kinouchi, Y. New water disinfection system using UVA light-emitting diodes. J. Appl. Microbiol. 2007, 103, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hirota, K.; Yumoto, H.; Matsuo, T.; Miyake, Y.; Ichikawa, T. Enhanced germicidal effects of pulsed UV-LED irradiation on biofilms. J. Appl. Microbiol. 2010, 109, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Birmpa, A.; Vantarakis, A.; Paparrodopoulos, S.; Whyte, P.; Lyng, J. Efficacy of three light technologies for reducing microbial populations in liquid suspensions. Biomed Res. Int. 2014, 2014, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Prasad, A.J.; Gänzle, M.; Roopesh, M.S. Inactivation of Salmonella spp. in wheat flour by 395 nm pulsed light emitting diode (LED) treatment and the related functional and structural changes of gluten. Food Res. Int. 2020, 127, 108716. [Google Scholar] [CrossRef]
- Kim, M.J.; Adeline Ng, B.X.; Zwe, Y.H.; Yuk, H.G. Photodynamic inactivation of Salmonella enterica Enteritidis by 405 ± 5-nm light-emitting diode and its application to control salmonellosis on cooked chicken. Food Control 2017, 82, 305–315. [Google Scholar] [CrossRef]
- Kumar, A.; Ghate, V.; Kim, M.J.; Zhou, W.; Khoo, G.H.; Yuk, H.G. Antibacterial efficacy of 405, 460 and 520 nm light emitting diodes on Lactobacillus plantarum, Staphylococcus aureus and Vibrio parahaemolyticus. J. Appl. Microbiol. 2016, 120, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Mercer, R.G.; Zheng, J.; Garcia-Hernandez, R.; Ruan, L.; Gänzle, M.G.; McMullen, L.M. Genetic determinants of heat resistance in Escherichia coli. Front. Microbiol. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mercer, R.G.; Walker, B.D.; Yang, X.; McMullen, L.M.; Gänzle, M.G. The locus of heat resistance (LHR) mediates heat resistance in Salmonella enterica, Escherichia coli and Enterobacter cloacae. Food Microbiol. 2017, 64, 96–103. [Google Scholar] [CrossRef]
- Uesugi, A.R.; Danyluk, M.D.; Harris, L.J. Survival of Salmonella Enteritidis phage type 30 on inoculated almonds stored at −20, 4, 23, and 35 °C. J. Food Prot. 2006, 69, 1851–1857. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Temelli, F.; Gänzle, M.G. Mechanisms of inactivation of dry Escherichia coli by high-pressure carbon dioxide. Appl. Environ. Microbiol. 2017, 83, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Syamaladevi, R.M.; Tang, J.; Zhong, Q. Water diffusion from a bacterial cell in low-moisture foods. J. Food Sci. 2016, 81, 2129–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blahovec, J.; Yanniotis, S. GAB generalized equation for sorption phenomena. Food Bioprocess Technol. 2008, 1, 82–90. [Google Scholar] [CrossRef]
- Quirijns, E.J.; Van Boxtel, A.J.B.; Van Loon, W.K.P.; Van Straten, G. Sorption isotherms, GAB parameters and isosteric heat of sorption. J. Sci. Food Agric. 2005, 85, 1805–1814. [Google Scholar] [CrossRef]
- Ghate, V.; Leong, A.L.; Kumar, A.; Bang, W.S.; Zhou, W.; Yuk, H.G. Enhancing the antibacterial effect of 461 and 521nm light emitting diodes on selected foodborne pathogens in trypticase soy broth by acidic and alkaline pH conditions. Food Microbiol. 2015, 48, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Shachar, D.; Yaron, S. Heat tolerance of Salmonella enterica serovars Agona, Enteritidis, and Typhimurium in peanut butter. J. Food Prot. 2006, 69, 2687–2691. [Google Scholar] [CrossRef]
- Kim, M.J.; Bang, W.S.; Yuk, H.G. 405 ± 5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiol. 2017, 62, 124–132. [Google Scholar] [CrossRef]
- Li, X.; Kim, M.-J.; Bang, W.-S.; Yuk, H.-G. Anti-biofilm effect of 405-nm LEDs against Listeria monocytogenes in simulated ready-to-eat fresh salmon storage conditions. Food Control 2018, 84, 513–521. [Google Scholar] [CrossRef]
- Kim, M.J.; Tang, C.H.; Bang, W.S.; Yuk, H.G. Antibacterial effect of 405 ± 5 nm light emitting diode illumination against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella on the surface of fresh-cut mango and its influence on fruit quality. Int. J. Food Microbiol. 2017, 244, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Mampallil, D.; Sharma, M.; Sen, A.; Sinha, S. Beyond the coffee-ring effect: Pattern formation by wetting and spreading of drops. Phys. Rev. E 2018, 98, 043107. [Google Scholar] [CrossRef]
- Oliver, S.P.; Jayarao, B.M.; Almeida, R. A Foodborne pathogens in milk and the dairy farm environment: Food safety and public health implications. Foodborne Pathog. Dis. 2005, 2, 115–129. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-K.; Kang, D.-H. Elevated inactivation efficacy of a pulsed UVC light-emitting diode system for foodborne pathogens on selective media and food surfaces. Appl. Environ. Microbiol. 2018, 84, 1340–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacombe, A.; Niemira, B.A.; Sites, J.; Boyd, G.; Gurtler, J.B.; Tyrell, B.; Fleck, M. Reduction of bacterial pathogens and potential surrogates on the surface of almonds using high-intensity 405-nanometer light. J. Food Prot. 2016, 79, 1840–1845. [Google Scholar] [CrossRef] [PubMed]
- Mille, Y.; Beney, L.; Gervais, P. Viability of Escherichia coli after combined osmotic and thermal treatment: A plasma membrane implication. Biochim. Biophys. Acta Biomembr. 2002, 1567, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Simonin, H.; Beney, L.; Gervais, P. Sequence of occurring damages in yeast plasma membrane during dehydration and rehydration: Mechanisms of cell death. Biochim. Biophys. Acta 2007, 1768, 1600–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willford, J.; Mendonca, A.; Goodridge, L.D. Water pressure effectively reduces Salmonella enterica serovar enteritidis on the surface of raw almonds. J. Food Prot. 2008, 71, 825–829. [Google Scholar] [CrossRef] [PubMed]






| Treatment Time (min) | Power Levels | |||
|---|---|---|---|---|
| 20% * | 40% | 60% | 100% | |
| 10 | 9.6 | 19.2 | 28.9 | 48.1 |
| 20 | 19.8 | 39.6 | 59.2 | 99.0 |
| 30 | 30.3 | 60.6 | 90.9 | 151.5 |
| 45 | 46.3 | 92.8 | 139.1 | 231.8 |
| 60 | 62.6 | 125.4 | 188.1 | 313.4 |
| Treatment Time (min) | Power Levels | ||
|---|---|---|---|
| 20% | 40% | 60% | |
| 5 | 22.6 | 45.2 | 67.8 |
| 10 | 46.2 | 92.6 | 138.8 |
| 15 | 69.9 | 139.9 | 209.9 |
| 17 | 79.5 | 159.2 | 238.7 |
| 20 | 92.9 | 186.2 | 279.2 |
| 30 | 139.4 | 279.3 | 418.7 |
| 45 | 208.9 | 418.4 | 627.3 |
| 60 | 277.9 | 556.6 | 834.4 |
| Treatment Time (min) | Water Content (kg Water/kg Dry Solids) with 395 nm LED | Water Activity Values Determined from Desorption Isotherm with 395 nm LED | Water Content (kg Water/kg Dry Solids) with 365 nm LED | Water Activity Values Determined from Desorption Isotherm with 365 nm LED |
|---|---|---|---|---|
| 0 | 0.168 ± 0.0124 a | 0.74 ± 0.0133 a | 0.168 ± 0.0124 a | 0.74 ± 0.0133 a |
| 10 | 0.137 ± 0.0171 ab | 0.698 ± 0.2115 a | 0.154 ± 0.0069 ab | 0.675 ± 0.0204 bc |
| 20 | 0.113 ± 0.0243 bc | 0.594 ± 0.1058 ab | 0.155 ± 0.0093 ab | 0.678 ± 0.0254 bc |
| 30 | 0.131 ± 0.0162 abc | 0.677 ± 0.0574 a | 0.158 ± 0.0042 ab | 0.686 ±0.0113 b |
| 45 | 0.083 ± 0.0267 c | 0.374 ± 0.0566 b | 0.142 ± 0.0053 b | 0.637 ± 0.0184 c |
| 60 | 0.101 ± 0.0528 bc | 0.545 ± 0.2377 ab | 0.154 ± 0.0146 ab | 0.675 ± 0.0431 bc |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, A.; Gänzle, M.; Roopesh, M.S. Inactivation of Escherichia Coli and Salmonella Using 365 and 395 nm High Intensity Pulsed Light Emitting Diodes. Foods 2019, 8, 679. https://doi.org/10.3390/foods8120679
Prasad A, Gänzle M, Roopesh MS. Inactivation of Escherichia Coli and Salmonella Using 365 and 395 nm High Intensity Pulsed Light Emitting Diodes. Foods. 2019; 8(12):679. https://doi.org/10.3390/foods8120679
Chicago/Turabian StylePrasad, Amritha, Michael Gänzle, and M. S. Roopesh. 2019. "Inactivation of Escherichia Coli and Salmonella Using 365 and 395 nm High Intensity Pulsed Light Emitting Diodes" Foods 8, no. 12: 679. https://doi.org/10.3390/foods8120679