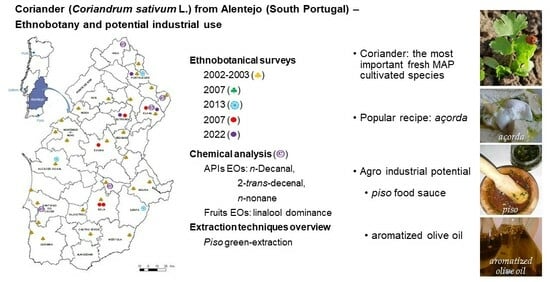
Coriander (Coriandrum sativum L.) from Alentejo (South Portugal)—Ethnobotany and Potential Industrial Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethnobotanical Surveys: Food Seasoning with Coriander in Alentejo Region
2.1.1. Surveys Conducted in 2002 and 2003
2.1.2. Surveys Conducted in 2013
2.2. Ethnobotanical Surveys: Coriander Representativeness on Local Markets
2.2.1. Surveys Conducted in 2007
2.2.2. Surveys Conducted in 2022
2.3. Chemical Characterization of Coriander Accessions
2.3.1. Essential Oils Isolation and Analysis
2.3.2. Fatty Acids Isolation and Analysis
2.4. Numeric Data Statistical Treatment
3. Results and Discussion
3.1. Ethnobotanical Surveys: Food Seasoning with Coriander in Alentejo Region
3.1.1. Surveys Conducted in 2002–2003
3.1.2. Surveys Conducted in 2013
3.1.3. Surveys Conducted in 2002–2003 and 2013
3.2. Coriander Representativeness on Local Markets
3.2.1. Surveys Conducted in 2007
3.2.2. Surveys Conducted in 2022
3.2.3. Surveys Conducted in 2007 and 2022
3.3. Coriander Aerial Parts and Fruits Essential Oils
3.4. Coriander Fruits Fatty Acids
3.5. Advances in Coriander Extraction Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camejo-Rodrigues, J.; Ascensão, L.; Bonet, M.A.; Vallès, J. An ethnobotanical study of medicinal and aromatic plants in the Natural Park of “Serra de São Mamede” (Portugal). J. Ethnopharmacol. 2003, 89, 199–209. [Google Scholar] [CrossRef]
- Silva, F.; Domingues, F.C. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit. Rev. Food Sci. Nutr. 2017, 57, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Sahib, N.G.; Anwar, F.; Gilani, A.H.; Hamid, A.A.; Saari, N.; Alkharfy, K.M. Coriander (Coriandrum sativum L.): A potential source of high-value components for functional foods and nutraceuticals--A review. Phytother. Res. 2013, 27, 1439–1456. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.A.; Dever, J.T.; Gafner, S.; Griffiths, J.C.; Marsman, D.S.; Rider, C.; Welch, C.; Embry, M.R. The Botanical Safety Consortium: A public-private partnership to enhance the botanical safety toolkit. Regul. Toxicol. Pharmacol. 2022, 128, 105090. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Hošek, J. Plants: From Farm to Food and Biomedical Applications. Appl. Sci. 2022, 12, 2803. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Ijaz, F.; Ali, N.; Shah, M.; Sana, U.; Bussmann, R.W. Historical perspectives of ethnobotany. Clin. Dermatol. 2019, 37, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Pranskuniene, Z.; Bajoraite, R.; Simaitiene, Z.; Bernatoniene, J. Home Gardens as a Source of Medicinal, Herbal and Food Preparations: Modern and Historical Approaches in Lithuania. Appl. Sci. 2021, 11, 9988. [Google Scholar] [CrossRef]
- United Nations. Transforming our World: The 2030 Agenda for Sustainable Development. 2125–2030 Agenda for Sustainable Development. United Nation. 2015. Available online: https://sustainabledevelopment.un.org/ (accessed on 24 July 2023).
- Ramadan, M.F. Handbook of Coriander (Coriandrum sativum): Chemistry, Functionality, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023; p. 590. [Google Scholar] [CrossRef]
- Zuzarte, M.; Girão, H.; Salgueiro, L. Aromatic Plant-Based Functional Foods: A Natural Approach to Manage Cardiovascular Diseases. Molecules 2023, 28, 5130. [Google Scholar] [CrossRef] [PubMed]
- Centre for the Promotion of Imports from Developing Countries (CBI). What Is the Demand for Natural Ingredients for Health Products on the European Market? Netherlands Enterprise Agency. 2022. Available online: https://www.cbi.eu/market-information/natural-ingredients-health-products/what-demand (accessed on 18 February 2024).
- Nurzynska-Wierdak, R. Chemical Diversity, Yield, and Quality of Aromatic Plants. Agronomy 2023, 13, 1614. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Banadka, A.; Nandhini, M.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M. Essential Oil from Coriandrum sativum: A review on Its Phytochemistry and Biological Activity. Molecules 2023, 28, 696. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef]
- Ramkumar, T.R.; Karuppusamy, S. Plant Diversity and Ethnobotanical Knowledge of Spices and Condiments. In Bioprospecting of Plant Biodiversity for Industrial Molecules; Chapter 12; Upadhyay, S.K., Singh, S.P., Eds.; John Wiley & Sons: West Sussex, UK, 2021; pp. 231–260. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Coriander (Coriandrum sativum): A promising functional food toward the well-being. Food Res. Int. 2018, 105, 305–323. [Google Scholar] [CrossRef]
- Kaur, H.; Patel, R.; Kaur, J. Ethnobotanical Knowledge of Umbelliferae Family: A Review. Int. J. Pharm. Sci. Invent. 2023, 12, 45–54. [Google Scholar]
- Souza, A.; Rodrigues, A.R.P.; Ribeiro, M.N.; Antunes, V.C. A study on the consumption of coriander (Coriandrum sativum L.) and consumers’ perception of its functional properties. Res. Soc. Dev. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Kajal, A.; Singh, R. Coriandrum sativum seeds extract mitigate progression of diabetic nephropathy in experimental rats via AGEs inhibition. PLoS ONE 2019, 14, e0213147. [Google Scholar] [CrossRef]
- Mahleyuddin, N.N.; Moshawih, S.; Ming, L.C.; Zulkifly, H.H.; Kifli, N.; Loy, M.J.; Sarker, M.M.R.; Al-Worafi, Y.M.; Goh, B.H.; Thuraisingam, S.; et al. Coriandrum sativum L.: A Review on Ethnopharmacology, Phytochemistry, and Cardiovascular Benefits. Molecules 2022, 27, 209. [Google Scholar] [CrossRef]
- Dias, A.S.; Dias, L.S. Herbs and spices in traditional Portuguese culinary: A preliminary picture. In VIII International Symposium of Ethnobotany 2010 Proceedings; Faculty of Pharmacy, University of Lisbon and Friends, the University for Peace Foundation: Lisbon, Portugal, 2011; ISBN 978-989-20-2789-0:595-602. Available online: http://hdl.handle.net/10174/3828 (accessed on 24 July 2023).
- Lopes, A.; Teixeira, D.; Calhau, C.; Pestana, D.; Padrão, P.; Graça, P. Ervas Aromáticas: Uma Estratégia para a Redução de sal na Alimentação dos Portugueses; DGS: Lisbon, Portugal, 2015; pp. 3–17. ISBN 978-972-675-223-3. Available online: https://repositorio-aberto.up.pt/bitstream/10216/82620/2/116245.pdf (accessed on 22 November 2023).
- Carvalho, A.M.; Pardo-de-Santayana, M.; Morales, R. Traditional knowledge of basketry practices in a Northeastern Region of Portugal. In Proceedings of the Fourth International Congress of Ethnobotany (ICEB 2005), Istanbul, Turkey, 21–26 August 2006; Available online: http://hdl.handle.net/10198/915 (accessed on 24 July 2023).
- Dias, A.S.; Dias, L.S. Herbs and spices in traditional recipes of Alentejo (Portugal). In Proceedings of the Fourth International Congress of Ethnobotany (ICEB 2005), Istanbul, Turkey, 21–26 August 2005; Ertug, Z.F., Ed.; Ege Yayinlari: Istanbul, Turkey, 2006; pp. 63–69. Available online: http://hdl.handle.net/10174/1355 (accessed on 24 July 2023).
- Moreira, C.; Farinha, N.; Póvoa, O. Preliminary Study of Coriander’s (Coriandrum sativum L.) Ethnobotany and Variability in the Alentejo Region. Rev. Fitoter. 2005, 5, 196–201. Available online: https://www.researchgate.net/publication/375863433 (accessed on 22 November 2023).
- Santos, S.; Correia, A.I.D.; Figueiredo, A.C.; Dias, L.S.; Dias, A.S. The use of herbal remedies in urban and rural areas of the Setubal Peninsula (Portugal): A study among elders. In Proceedings of the Fourth International Congress of Ethnobotany (ICEB 2005), Istanbul, Turkey, 21–26 August 2005; Ertug, Z.F., Ed.; Ege Yayinlari: Istanbul, Turkey, 2006; pp. 197–201. Available online: http://hdl.handle.net/10174/1356 (accessed on 24 July 2023).
- Póvoa, O.; Ribeiro, G.; Rodrigues, L.; Lobato, P.; Monteiro, P.; Monteiro, A.; Moldão-Martins, M. Effect of storage time on physico-chemical characteristics of Mentha pulegium L. and Mentha cervina L. Piso Food Sauce. Acta Hortic. 2009, 826, 193–200. [Google Scholar] [CrossRef]
- Lopes, E.; Farinha, N.; Póvoa, O. Characterization and evaluation of traditional and wild coriander in Alentejo (Portugal). Acta Hortic. 2017, 1153, 77–84. [Google Scholar] [CrossRef]
- Póvoa, O.; Lopes, V.; Barata, A.M.; Farinha, N. Monitoring Genetic Erosion of Aromatic and Medicinal Plant Species in Alentejo (South Portugal). Plants 2023, 12, 2588. [Google Scholar] [CrossRef] [PubMed]
- Farinha, N.; Churra, M.; Paulo, M.; Lopes, E.; Barata, A.; Lopes, V.; Figueiredo, A.C.; Serrano, C.; Póvoa, O. Plant breeding of Coriandrum sativum from landraces collected in Alentejo (Portugal). Acta Hortic. 2023, 1358, 57–64. [Google Scholar] [CrossRef]
- Póvoa, O.; Farinha, N.; Dias, S.S. Levantamento Etnobotânico Sobre Coentros e Poejos no Alentejo. 2012. Available online: http://hdl.handle.net/10400.26/4598 (accessed on 3 July 2023).
- Farinha, N.; Póvoa, O. Participação da Escola Superior Agrária de Elvas no Projecto AGRO 34 “Etnobotânica, o Uso e a Gestão das Plantas Aromáticas e Medicinais e a sua Utilização Sustentável como Contributo para a Valorização do Meio Rural”. 2005. Available online: https://www.researchgate.net/publication/375828496 (accessed on 25 November 2023).
- Dinis, S.C.B. Recolha de Conhecimentos Etnobotânicos Aplicados ao Tratamento de Animais em três Aldeias do Alentejo. 2014. Available online: https://comum.rcaap.pt/handle/10400.26/9773 (accessed on 9 June 2023).
- Monteiro, A.; Póvoa, O.; Pereira, J.; Afonso, E. Conservação de germoplasma, produção e utilização dos taxa Mentha pulegium, Mentha cervina e Thymbra capitata. In Relatório final do Projecto AGRO 522; Instituto Superior de Agronomia, Universidade de Lisboa: Lisboa, Portugal, 2008. [Google Scholar] [CrossRef]
- Póvoa, O.; Paulo, M.; Rodrigues, F.; Churra, M.; Santana, C.; Conceição, L.; Farinha, N. Orégãos do Alentejo. In Do campo ao prato; IPP: Portalegre, Portugal, 2022; ISBN 978-989-8806-59-8. [Google Scholar]
- Council of Europe; European Pharmacopoeia Commission; European Directorate for the Quality of Medicines & Healthcare. European Directorate for the Quality of Medicines. In European Pharmacopoeia, 7th ed.; Council of Europe, European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2010; p. 241. [Google Scholar]
- ISO 7609:1985; Essential Oils-Analysis by Gas Chromatography on Capillary Columns-General Method. ISO: Geneva, Switzerland, 1985.
- Santos, P.M.; Figueiredo, A.C.; Oliveira, M.M.; Barroso, J.G.; Pedro, L.G.; Deans, S.G.; Younus, A.K.M.; Scheffer, J.J.C. Essential Oils from Hairy Root Cultures and from Fruits and Roots of Pimpinella anisum. Phytochemistry 1998, 48, 455–460. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Sim-Sim, M.; Barroso, J.G.; Pedro, L.G.; Santos, P.A.G.; Fontinha, S.S.; Schripsema, J.; Deans, S.G.; Scheffer, J.J.C. Composition of the Essential Oil From the Liverwort Marchesinia mackaii (Hook.) S. F. Gray Grown in Portugal. J. Essent. Oil Res. 2002, 14, 439–442. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pais, M.S.S.; Scheffer, J.J.C. Composition of the Essential Oils from Leaves and Flowers of Achillea millefolium L. ssp. millefolium. Flavour Fragr. J. 1992, 7, 219–222. [Google Scholar] [CrossRef]
- Ascensão, L.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Schripsema, J.; Deans, S.G.; Scheffer, J.J.C. Plectranthus madagascariensis: Morphology of the Glandular Trichomes, Essential Oil Composition, and Its Biological Activity. Int. J. Plant Sci. 1998, 159, 31–38. [Google Scholar] [CrossRef]
- Santos, P.A.G.; Figueiredo, A.C.; Lourenço, P.M.L.; Barroso, J.G.; Pedro, L.G.; Oliveira, M.M.; Schripsema, J.; Deans, S.G.; Scheffer, J.J.C. Hairy Root Cultures of Anethum graveolens (dill): Establishment, Growth, Time-Course Study of Their Essential Oil and Its Comparison with Parent Plant Oils. Biotechnol. Lett. 2002, 24, 1031–1036. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Sena, I.; Moiteiro, C.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Nematotoxic and Phytotoxic Activity of Satureja montana and Ruta graveolens Essential Oils on Pinus pinaster Shoot Cultures and P. pinaster with Bursaphelenchus xylophilus in vitro Co-cultures. Ind. Crops Prod. 2015, 77, 59–65. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Rodrigues, A.M.; Sena, I.; Moiteiro, C.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity of Ruta graveolens and Satureja montana Essential Oils on Solanum tuberosum Hairy Roots and Solanum tuberosum Hairy Roots with Meloidogyne chitwoodi Co-cultures. J. Agric. Food Chem. 2016, 64, 7452–7458. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, L.R.; Vila, R.; Tomi, F.; Figueiredo, A.C.; Barroso, J.G.; Cañigueral, S.; Casanova, J.; Proença da Cunha, A.; Adzet, T. Variability of Essential Oils of Thymus caespititius from Portugal. Phytochemistry 1997, 45, 307–311. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Salgueiro, L.; Barroso, J.G.; Figueiredo, A.C.; Pedro, L.G.; Fontinha, S.S.; Bighelli, A.; Casanova, J.; Looman, A.; Scheffer, J.J.C. Composition of the Essential Oil of Juniperus cedrus Webb & Berth. Grown on Madeira. Flavour Fragr. J. 2002, 17, 111–114. [Google Scholar]
- Figueiredo, A.C.; Moiteiro, C.; Rodrigues, M.C.S.M.; Almeida, A.J.R.M. Essential Oil Composition from Cryptomeria japonica D.Don Grown in Azores: Biomass Valorization from Forest Management. Nat. Prod. Commun. 2021, 16, 1934578X211038431. [Google Scholar] [CrossRef]
- Machado, A.M.; Lopes, V.; Barata, A.M.; Póvoa, O.; Farinha, N.; Figueiredo, A.C. Chemical Variability of the Essential Oils from Two Portuguese Apiaceae: Coriandrum sativum L. and Foeniculum vulgare Mill. Plants 2023, 12, 2749. [Google Scholar] [CrossRef]
- Microsoft. Microsoft Excel 2016; Microsoft Corporation: Redmond, WA, USA, 2016. [Google Scholar]
- Rohlf, J.F. NTSYS-pc, Numerical Taxonomy and Multivariate Analysis System; Version 2.1; User Guide and Exeter Publishing Setauket: New York, NY, USA, 2000. [Google Scholar]
- Pestana, M.H.; Gageiro, J.N. Análise De Dados Para Ciências Sociais: A Complementaridade do SPSS; Edições Sílabo: Lisboa, Portugal, 2000. [Google Scholar]
- Gabinete de Planeamento e Políticas. As Plantas Aromáticas Medicinais e Condimentares, Portugal Continental 2012. Ministério da Agricultura e do Mar. 2013. Available online: https://www.gpp.pt/images/GPP/O_que_disponibilizamos/Publicacoes/Estudo_PAM_final.pdf (accessed on 24 January 2024).
- ISO 2255:1996; Coriander (Coriandrum sativum L.), whole or ground (powdered). Last Reviewed and Confirmed in 2018. ISO: Geneva, Switzerland, 2018.
- 2014/155/EU; The Publications Office of the European Union, Commission Implementing Decision of 19 March 2014 Authorising the Placing on the Market of Coriander Seed Oil as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (notified under document C (2014). Official Journal of the European Union, L 85, 21.3.2014, p. 13–14. The Publications Office of the European Union: Luxembourg, 1689.
- Uitterhaegen, E.; Nguyen, Q.H.; Sampaio, K.A.; Stevens, C.V.; Merah, O.; Talou, T.; Rigal, L.; Evon, P. Extraction of Coriander Oil Using Twin Screw Extrusion: Feasibility Study and Potential Press Cake Applications. J. Am. Oil Chem. Soc. 2015, 92, 1219–1233. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J.-T. Oil composition of coriander (Coriandrum sativum L.) fruit-seeds. Eur. Food Res. Technol. 2002, 215, 204–209. [Google Scholar] [CrossRef]
- Sriti, J.; Aidi Wannes, W.; Talou, T.; Mhamdi, B.; Hamdaoui, G.; Marzouk, B. Lipid, fatty acid and tocol distribution of coriander fruit’s different parts. Ind. Crops Prod. 2010, 31, 294–300. [Google Scholar] [CrossRef]
- ISO 3516:1997; ISO and Oil of Coriander Fruits (Coriandrum sativum L.). Last Reviewed and Confirmed in 2023. ISO: Geneva, Switzerland, 2023.
- European Pharmacopoeia (Ph. Eur.) 10th Edition-European Directorate for the Quality of Medicines & HealthCare-EDQM. Available online: https://www.edqm.eu/en/ (accessed on 8 November 2023).
- Instituto Nacional da Farmácia e do Medicamento. Farmacopeia Portuguesa 9.0; Autoridade Nacional do Medicamento e Produtos de Saúde: Lisboa, Portugal, 2008. [Google Scholar]
- European Medicines Agency: EMA/HMPC/180400/2018. European Union Herbal Monograph on Species Digestivae; EMA: Amsterdam, The Netherlands, 2022; Available online: https://docplayer.net/234480337-European-union-herbal-monograph-on-species-digestivae.html (accessed on 22 November 2023).
- Pavlić, B.; Vidović, S.; Vladić, J.; Radosavljević, R.; Zeković, Z. Isolation of coriander (Coriandrum sativum L.) essential oil by green extractions versus traditional techniques. J. Supercrit. Fluids 2015, 99, 23–28. [Google Scholar] [CrossRef]
- Grosso, C.; Ferraro, V.; Figueiredo, A.C.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M. Supercritical carbon dioxide extraction of volatile oil from Italian coriander seeds. Food Chem. 2008, 111, 197–203. [Google Scholar] [CrossRef]
- Ghazanfari, N.; Mortazavi, S.A.; Yazdi, F.T.; Mohammadi, M. Microwave-assisted hydrodistillation extraction of essential oil from coriander seeds and evaluation of their composition, antioxidant and antimicrobial activity. Heliyon 2020, 6, e04893. [Google Scholar] [CrossRef] [PubMed]
- Kaanin-Boudraa, G.; Brahmi, F.; Wrona, M.; Nerín, C.; Moudache, M.; Mouhoubi, K.; Madani, K.; Boulekbache-Makhlouf, L. Response surface methodology and UPLC-QTOF-MSE analysis of phenolic compounds from grapefruit (Citrus × paradisi) by-products as novel ingredients for new antioxidant packaging. LWT 2021, 151, 112158. [Google Scholar] [CrossRef]
- Mouhoubi, K.; Boulekbache-Makhlouf, L.; Madani, K.; Freidja, M.L.; Silva, A.M.; Cardoso, S.M. Microwave-assisted extraction optimization and conventional extraction of phenolic compounds from coriander leaves: UHPLC characterization and antioxidant activity. North Afr. J. Food Nutr. Res. 2023, 7, 69–83. [Google Scholar] [CrossRef]
- Scandar, S.; Zadra, C.; Lanari, D.; Marcotullio, M.C. Boosting the essential oil yield of Coriandrum sativum L. using choline chloride-based NADES: An optimized and systematic study employing response surface methodology (RSM). Ind. Crops Prod. 2024, 208, 117920. [Google Scholar] [CrossRef]
- Shahwar, M.K.; El-Ghorab, A.H.; Anjum, F.M.; Butt, M.S.; Hussain, S.; Nadeem, M. Characterization of coriander (Coriandrum sativum L.) seeds and leaves: Volatile and non volatile extracts. Int. J. Food Prop. 2012, 15, 736–747. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmad, R.; Saeed, S.; Azeem, M.; Mozūraitis, R.; Borg-Karlson, A.K.; Zhu, G. Chemical composition of fresh leaves headspace aroma and essential oils of four Coriander cultivars. Front. Plant Sci. 2022, 13, 820644. [Google Scholar] [CrossRef] [PubMed]
- Eikani, M.H.; Golmohammad, F.; Rowshanzamir, S. Subcritical water extraction of essential oils from coriander seeds (Coriandrum sativum L.). J. Food Eng. 2007, 80, 735–740. [Google Scholar] [CrossRef]
- Msaada, K.; Taârit, M.B.; Hosni, K.; Salem, N.; Tammar, S.; Bettaieb, I.; Hammami, M.; Limam, F.; Marzouk, B. Comparison of different extraction methods for the determination of essential oils and related compounds from coriander (Coriandrum sativum L.). Acta Chim. Slov. 2012, 59, 803–813. [Google Scholar] [PubMed]
- Sriti, J.; Talou, T.; Faye, M.; Vilarem, G.; Marzouk, B. Oil extraction from coriander fruits by extrusion and comparison with solvent extraction processes. Ind. Crops Prod. 2011, 33, 659–664. [Google Scholar] [CrossRef]
- Song, E.J.; Ko, M.J. Extraction of monoterpenes from coriander (Coriandrum sativum L.) seeds using subcritical water extraction (SWE) technique. J. Supercrit. Fluids 2022, 188, 105668. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Marin, F.R.; Herrero, M.; Señorans, F.J.; Reglero, G.; Cifuentes, A.; Ibáñez, E. Subcritical water ex-traction of nutraceuticals with antioxidant activity from oregano. Chemical and functional characterization. J. Pharm. Biomed. Anal. 2006, 41, 1560–1565. [Google Scholar] [CrossRef]
- Abbas, A.; Anwar, F.; Ahmad, N.; Shahid, M.; Al-Mijalli, S.H.; Yaseen, M.; Farooq, S.; Iqbal, M. Characterization of bioactives and nutra-pharmaceutical potential of supercritical fluid and hydro-distilled extracted coriander leaves essential oil. Dose-Response 2022, 20, 15593258221130749. [Google Scholar] [CrossRef]
- Wei, S.; Lyu, J.; Wei, L.; Xie, B.; Wei, J.; Zhang, G.; Li, J.; Girão, H.; Salgueiro, L.; Yu, J. Chemometric approaches for the optimization of headspace-solid phase microextraction to analyze volatile compounds in coriander (Coriandrum sativum L.). LWT 2022, 167, 113842. [Google Scholar] [CrossRef]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Kassahun, B.M. Unleashing the exploitation of coriander (Coriander sativum L.) for biological, industrial and pharmaceutical applications. J. Agric. Sci. Res. 2020, 8, 552–564. [Google Scholar]
- Póvoa, O.; Farinha, N.; Lopes, E.; Mendes, J.P.; Pereira, L. Coentros do Alentejo–Conservação do Conhecimento Tradicional e dos Recursos Genéticos; C3i do Instituto Politécnico de Portalegre/77’83 Atelier de Design: Portalegre, Portugal, 2014; ISBN 978-989-8806-00-0. [Google Scholar]










| Sample Code | BPGV Accession | Accession Origin: Municipality/District | Plant Part | Yield (%, v/v) |
| Essential oil Analysis | ||||
| Cs_1_API_14 | BPGV08514 | Elvas, Portalegre | APIs | 0.04 |
| Cs_16_API_14 | BPGV19284 | Vidigueira, Beja | APIs | 0.05 |
| Cs_32_API_14 | BPGV28149 | Campo Maior, Portalegre | APIs | 0.05 |
| Cs_TP_API_14 | Commercial variety | APIs | 0.04 | |
| Cs_1_F_14 | BPGV08514 | Elvas, Portalegre | Fruits | 0.46 |
| Cs_16_F_14 | BPGV19284 | Vidigueira, Beja | Fruits | 0.39 |
| Cs_26_F_13 | BPGV08524 | Santiago do Cacém, Setúbal | Fruits | 0.25 |
| Cs_31_F_14 | BPGV19293 | Campo Maior, Portalegre | Fruits | 0.25 |
| Cs_31_F_14_w | BPGV19293 | Campo Maior, Portalegre | Fruits | 0.14 |
| Cs_32_F_14 | BPGV28149 | Campo Maior, Portalegre | Fruits | 0.37 |
| Cs_TP_F_14 | Commercial variety | Fruits | 0.40 | |
| Sample Code | BPGV Accession | Accession Origin Municipality/District | Plant Part | Yield (%, w/w) |
| Fatty Acids Analysis | ||||
| Cs_1_1_F_14 | BPGV08514 | Elvas, Portalegre | Fruits | 0.8 |
| Cs_1_2_F_14 | BPGV08514 | Elvas, Portalegre | Fruits | 0.9 |
| Cs_1_3_F_14 | BPGV08514 | Elvas, Portalegre | Fruits | 1.2 |
| Cs_16_1_F_14 | BPGV19284 | Vidigueira, Beja | Fruits | 1.2 |
| Cs_16_2_F_14 | BPGV19284 | Vidigueira, Beja | Fruits | 0.7 |
| Cs_16_3_F_14 | BPGV19284 | Vidigueira, Beja | Fruits | 0.8 |
| Cs_31_F_14_w | BPGV19293 | Campo Maior, Portalegre | Fruits | 0.8 |
| Cs_32_1_F_14 | BPGV28149 | Campo Maior, Portalegre | Fruits | 1.5 |
| Cs_32_2_F_14 | BPGV28149 | Campo Maior, Portalegre | Fruits | 1.1 |
| Cs_32_3_F_14 | BPGV28149 | Campo Maior, Portalegre | Fruits | 1.2 |
| Cs_33_F_14_w | BPGV19649 | Campo Maior, Portalegre | Fruits | 0.7 |
| Cs_Santo_1_F_14 | Commercial variety | Fruits | 1.0 | |
| Cs_Santo_2_F_14 | Commercial variety | Fruits | 0.9 | |
| Cs_Santo_3_F_14 | Commercial variety | Fruits | 1.5 | |
| Cs_TP_1_F_14 | Commercial variety | Fruits | 0.5 | |
| Cs_TP_2_F_14 | Commercial variety | Fruits | 0.8 | |
| Cs_TP_3_F_14 | Commercial variety | Fruits | 0.5 |
| Recipe Type | Alegrete | Santa Catarina | Vale de Vargo | Total 3 Villages |
|---|---|---|---|---|
| Açorda * | 20 | 20 | 20 | 60 |
| Fish | 18 | 20 | 13 | 51 |
| Rice | 16 | 20 | 15 | 51 |
| Fabaceae | 11 | 18 | 17 | 46 |
| Fish soup | 18 | 19 | 7 | 44 |
| Meat | 18 | 11 | 8 | 37 |
| Piso * | 16 | 15 | 1 | 32 |
| Migas * | 2 | 6 | 0 | 8 |
| Total | 119 | 129 | 81 | 329 |
| Recipe Type | Surveys | ||
|---|---|---|---|
| 2003 | 2013 | Total | |
| Fabaceae | 19 | 17 | 36 |
| Açorda * and migas * | 24 | 5 | 29 |
| Fish | 7 | 19 | 26 |
| Other recipes | 8 | 16 | 24 |
| Meat | 4 | 6 | 10 |
| Total | 62 | 63 | 125 |
| Peak | APIs | Fruits | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Components | RI | Cs_1_ API_14 | Cs_16_API_14 | Cs_32_API_14 | Cs_TP_API_14 | Cs_1_F_14 | Cs_16_F_14 | Cs_26_F_13 | Cs_31_F_14 | Cs_31_F_14_w | Cs_32_F_14 | Cs_TP_F_14 |
| 1 | n-Hexanal | 756 | t | t | t | t | t | t | t | ||||
| 2 | n-Octane | 800 | 0.7 | 1.0 | 0.5 | 1.2 | |||||||
| 3 | cis-3-Hexen-1-ol | 868 | t | 0.2 | 0.1 | t | |||||||
| 4 | cis-2-Hexen-1-ol | 882 | t | 0.1 | 0.1 | t | |||||||
| 5 | n-Hexanol | 882 | t | 0.1 | t | t | |||||||
| 6 | n-Heptanal | 897 | t | t | t | t | t | t | 0.1 | 0.1 | t | t | t |
| 7 | 1-Nonene | 899 | t | t | t | t | |||||||
| 8 | n-Nonane | 900 | 15.1 | 9.9 | 11.0 | 17.2 | t | t | t | 0.1 | t | t | t |
| 9 | trans-2-Nonene * | 900 | t | t | t | t | |||||||
| 10 | α-Thujene | 924 | t | t | 0.1 | 0.1 | 0.1 | t | t | ||||
| 11 | α-Pinene | 930 | 0.5 | 0.4 | 0.4 | 0.6 | 4.6 | 3.3 | 3.0 | 8.7 | 6.2 | 3.6 | 4.1 |
| 12 | Camphene | 938 | 0.5 | 0.4 | 0.3 | 0.5 | 0.4 | 0.4 | 0.4 | ||||
| 13 | n-Heptanol | 952 | t | t | t | t | t | t | t | t | t | t | t |
| 14 | Sabinene | 958 | t | t | t | 0.1 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 |
| 15 | 1-Octen-3-ol | 961 | t | t | t | t | t | t | t | t | t | t | t |
| 16 | β-Pinene | 963 | t | t | t | t | 0.4 | t | 0.4 | 0.9 | 0.6 | 0.3 | 0.4 |
| 17 | 3-Methyl nonane | 970 | t | t | t | t | |||||||
| 18 | 2-Pentyl furan | 973 | t | t | 0.1 | t | t | t | t | ||||
| 19 | n-Octanal | 973 | 3.0 | 2.6 | 2.1 | 2.8 | t | t | 0.1 | t | t | t | t |
| 20 | β-Myrcene | 975 | 0.8 | 0.8 | 1.3 | 1.6 | 1.3 | 0.7 | 0.8 | ||||
| 21 | α-Phellandrenene | 995 | t | t | t | t | t | t | t | ||||
| 22 | n-Decane | 1000 | 1.2 | 0.6 | 0.7 | 0.8 | |||||||
| 23 | α-Terpinene | 1002 | t | t | 0.1 | 0.1 | 0.1 | t | 0.1 | ||||
| 24 | p-Cymene | 1003 | t | t | t | t | 0.9 | 1.1 | 1.1 | 0.6 | 1.0 | 1.2 | 1.6 |
| 25 | β-Phellandrene | 1005 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | ||||
| 26 | Limonene | 1009 | t | t | t | 0.1 | 1.4 | 1.4 | 1.4 | 1.6 | 1.3 | 1.2 | 1.4 |
| 27 | cis-β-Ocimene | 1017 | t | t | 0.2 | 0.2 | 0.2 | t | t | ||||
| 28 | trans-β-Ocimene | 1027 | t | t | t | t | t | t | 0.3 | 0.3 | 0.3 | t | t |
| 29 | γ-Terpinene | 1035 | t | t | t | 0.1 | 4.8 | 6.2 | 4.4 | 4.3 | 8.3 | 7.3 | 5.7 |
| 30 | trans-Sabinene hydrate | 1037 | t | t | t | t | t | t | t | ||||
| 31 | cis-Linalool oxide | 1045 | t | 0.3 | t | 0.1 | t | t | 0.1 | ||||
| 32 | n-Octanol | 1045 | 0.4 | 0.3 | 0.3 | 0.4 | t | t | t | t | t | t | t |
| 33 | trans-Linalool oxide | 1059 | t | t | 0.1 | t | t | t | 0.1 | ||||
| 34 | Terpinolene | 1064 | 0.3 | t | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | ||||
| 35 | n-Nonanal | 1073 | 0.9 | 1.0 | 1.0 | 1.0 | |||||||
| 36 | Linalool | 1074 | 0.2 | 0.5 | 0.2 | 0.1 | 77.9 | 75.7 | 78.0 | 73.3 | 73.4 | 73.7 | 77.7 |
| 37 | 1-Octen-3-yl acetate | 1086 | t | t | t | t | |||||||
| 38 | n-Undecane | 1100 | 0.2 | t | 0.1 | 0.1 | |||||||
| 39 | Camphor | 1102 | 2.9 | 4.3 | 2.1 | 2.3 | 1.9 | 3.9 | 2.7 | ||||
| 40 | Citronellal | 1121 | t | 0.1 | 0.6 | 0.9 | 0.1 | t | |||||
| 41 | 2-trans-Nonenal | 1124 | 0.1 | 0.2 | 0.2 | 0.1 | t | ||||||
| 42 | Borneol | 1134 | 0.3 | t | 0.6 | t | 0.1 | 0.1 | 0.1 | ||||
| 43 | Terpinen-4-ol | 1148 | 0.2 | 0.3 | 0.3 | 0.2 | 0.2 | 0.4 | 0.3 | ||||
| 44 | n-Nonanol | 1148 | 0.3 | 0.5 | 0.4 | 0.3 | |||||||
| 45 | α-Terpineol | 1159 | 0.3 | 0.4 | 0.3 | 0.2 | 0.2 | 0.3 | 0.3 | ||||
| 46 | 4-cis-Decenal | 1163 | 0.2 | 0.3 | 0.2 | 0.2 | |||||||
| 47 | n-Decanal | 1180 | 23.0 | 20.9 | 21.0 | 24.1 | 0.1 | 0.1 | 0.4 | 0.5 | 0.3 | 0.2 | 0.2 |
| 48 | Pulegone | 1207 | t | t | t | t | 0.1 | t | 0.1 | 0.1 | t | t | 0.1 |
| 49 | Citronellol | 1207 | t | t | t | t | 0.1 | t | 0.1 | 0.5 | 0.6 | 0.1 | t |
| 50 | Geraniol | 1236 | 2.0 | 3.0 | 2.0 | 0.8 | 0.6 | 3.3 | 1.4 | ||||
| 51 | 2-trans-Decenal | 1236 | 12.2 | 17.9 | 16.1 | 13.1 | |||||||
| 52 | 2-trans-Decen-1-ol * | 1256 | 9.2 | 9.8 | 8.8 | 7.5 | |||||||
| 53 | n-Decanol | 1259 | 6.7 | 6.5 | 6.0 | 7.1 | t | t | 0.1 | 0.1 | 0.1 | t | t |
| 54 | n-Undecanal | 1288 | 4.0 | 4.2 | 4.2 | 3.2 | |||||||
| 55 | Myrtenyl acetate | 1290 | t | t | 0.1 | 0.1 | t | 0.1 | t | ||||
| 56 | 2-trans-Undecenal | 1323 | 1.9 | 3.7 | 3.2 | 1.6 | |||||||
| 57 | 2-trans-Undecen-1-ol * | 1325 | 0.7 | 1.2 | 1.0 | 0.5 | |||||||
| 58 | Citronellyl acetate | 1343 | t | t | t | 0.1 | t | t | t | ||||
| 59 | Neryl acetate | 1353 | t | t | t | t | t | t | t | ||||
| 60 | n-Undecanol | 1366 | 0.5 | 0.6 | 0.5 | 0.4 | |||||||
| 61 | Geranyl acetate | 1370 | 1.3 | 1.1 | 1.8 | 1.0 | 0.8 | 0.8 | 1.6 | ||||
| 62 | n-Dodecanal | 1397 | 4.3 | 3.2 | 3.8 | 3.8 | t | t | 0.1 | t | t | t | t |
| 63 | 2-trans-Dodecenal | 1446 | 5.3 | 5.4 | 5.3 | 5.1 | 0.3 | t | 0.2 | 0.2 | 0.2 | 0.2 | t |
| 64 | 2-trans-Dodecen-1-ol | 1448 | 1.1 | 1.2 | 1.1 | 1.0 | |||||||
| 65 | n-Dodecanol | 1468 | 0.2 | 0.2 | 0.2 | 0.2 | |||||||
| 66 | α-Amorphene | 1476 | 0.1 | t | t | t | |||||||
| 67 | n-Tridecanal | 1499 | 0.4 | 0.3 | 0.3 | 0.4 | |||||||
| 68 | 2-trans-Tridecen-1-al | 1574 | 0.4 | 0.2 | 0.4 | 0.4 | |||||||
| 69 | n-Tetradecanal (=miristaldehyde) | 1596 | 0.7 | 0.6 | 0.9 | 0.7 | |||||||
| 70 | α-Cadinol | 1626 | 0.1 | t | t | t | |||||||
| 71 | cis-9-Tetradecenal * | 1647 | 2.9 | 2.7 | 3.4 | 2.9 | |||||||
| 72 | n-Pentadecanal | 1688 | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| 73 | 11-Pentadecenal * | 1735 | 0.5 | 0.4 | 0.4 | 0.5 | |||||||
| 74 | Hexadecanoic acid (=palmitic acid) | 1908 | t | t | t | t | |||||||
| 75 | Phytol acetate 2 | 2101 | 0.1 | t | t | t | |||||||
| 76 | Methyl linoleate | 2101 | 0.1 | t | t | t | |||||||
| 77 | n-Nonadecanal * | 2102 | t | t | t | t | |||||||
| 78 | Petroselinic acid | 2128 | t | t | t | t | t | t | t | ||||
| 79 | n-Eicosanal | 2200 | 0.1 | t | 0.1 | t | |||||||
| % Identification | 97.4 | 96.8 | 94.1 | 97.7 | 99.5 | 98.7 | 99.9 | 99.9 | 99.9 | 98.6 | 99.7 | ||
| Grouped components | |||||||||||||
| Monoterpene hydrocarbons | 0.5 | 0.4 | 0.4 | 0.9 | 14.0 | 13.5 | 13.2 | 19.6 | 20.6 | 15.4 | 15.1 | ||
| Oxygen-containing monoterpenes | 0.2 | 0.5 | 0.2 | 0.1 | 85.1 | 85.1 | 85.6 | 79.3 | 78.7 | 82.8 | 84.4 | ||
| Sesquiterpene hydrocarbons | 0.1 | t | t | t | |||||||||
| Oxygen-containing sesquiterpenes | 0.1 | t | t | t | |||||||||
| Oxygen-containing diterpenes | 0.1 | t | t | t | |||||||||
| Fatty acids | t | t | t | t | t | t | t | t | t | t | t | ||
| Fatty acid derivatives | 79.2 | 84.0 | 81.0 | 77.4 | 0.4 | 0.1 | 1.0 | 0.9 | 0.6 | 0.4 | 0.2 | ||
| Others | 17.2 | 11.7 | 12.4 | 19.3 | t | t | 0.1 | 0.1 | t | t | t |
| Peak # | FAMES | Fatty Acids | Formula | C:D | ω | Cs_1_F_14 | Cs_16_F_14 | Cs_31_F_14_w | Cs_32_F_14 | Cs_33_F_14_w | Cs_ Santo_F_14 | Cs_ TP_ F_14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Methyl dodecanoate (=Methyl laurate) | Dodecanoic acid (=lauric acid) | C12H24O2 | C12:0 | t | t | t | t | t | t | t | |
| 2 | Methyl tridecanoate | Tridecanoic acid (=tridecylic acid) | C13H26O2 | C13:0 | t | t | t | t | t | t | t | |
| 3 | Methyl tetradecanoate (=methyl myristate) | Tetradecanoic acid (=myristic acid) | C14H28O2 | C14:0 | 7.6 | 17.5 | 6.5 | 4.2 | 4.1 | 5.6 | 4.9 | |
| 4 | Methyl pentadecanoate | Pentadecanoic acid (=pentadecylic acid) | C15H30O2 | C15:0 | t | t | t | t | t | t | t | |
| 5 | cis-10-Heptadecenoic acid methyl ester | cis-10-Heptadecenoic acid | C17H32O2 | C17:1 cis-10 | ω 7 | t | t | t | t | t | t | t |
| 6 | Methyl cis-9-hexadecenoate (=methyl palmitoleate) | cis-9-Hexadecenoic acid (=palmitoleic acid) | C16H30O2 | C16:1 cis-9 | ω 7 | t | t | t | t | t | t | t |
| 7 | Methyl hexadecanoate (=methyl palmitate) | Hexadecanoic acid (=palmitic acid) | C16H32O2 | C16:0 | 19.2 | 29.7 | 27.3 | 12.9 | 22.3 | 14.7 | 12.0 | |
| 8 | Methyl heptadecanoate (=methyl margarate) | Heptadecanoic acid (=margaric acid) | C17H34O2 | C17:0 | t | t | t | t | t | t | t | |
| 9 | Methyl cis,cis-9,12-octadecadienoate (=methyl linoleate) | (9Z,12Z)-9,12-Octadecadienoic acid (=linoleic acid, grape seed oil) | C18H32O2 | C18:2 cis-9, cis-12 | ω 6 | 16.7 | 14.0 | 7.6 | 19.1 | 12.3 | 18.3 | 19.2 |
| 10 | Methyl cis-6-octadecenoate (=methyl petroselinate) | (6Z)-Octadec-6-enoic acid (=petroselinic acid) | C18H34O2 | C18:1 cis-6 | ω 12 | 45.6 | 31.5 | 45.1 | 55.3 | 54.1 | 54.7 | 55.4 |
| 11 | Methyl cis-9-octadecenoate (=methyl oleate) | (9Z)-Octadec-9-enoic acid (=oleic acid) | C18H34O2 | C18:1 cis-9 | ω 9 | t | t | t | t | t | t | t |
| 12 | Methyl octadecanoate (=methyl stearate) | Octadecanoic acid (=stearic acid) | C18H36O2 | C18:0 | 3.9 | 4.6 | 8.1 | 2.7 | 1.7 | 2.8 | 2.1 | |
| 13 | Methyl eicosanoate (=methyl arachidate) | Eicosanoic acid (=arachidic acid) | C20H40O2 | C20:0 | t | t | t | t | t | t | t | |
| % of identification | 93.1 | 97.3 | 94.6 | 94.2 | 94.5 | 96.2 | 93.6 | |||||
| Fatty acids ratio | ||||||||||||
| Petroselinic acid:Myristic acid | (C18:1 cis-6):(C14:0) | 6.1 | 1.8 | 6.9 | 13.1 | 13.2 | 9.8 | 11.6 | ||||
| Petroselinic acid:Palmitic acid | (C18:1 cis-6):(C16:0) | 2.4 | 1.1 | 1.7 | 4.3 | 2.4 | 3.8 | 4.8 | ||||
| Petroselinic acid:Linoleic acid | (C18:1 cis-6):(C18:2) | 2.7 | 2.3 | 5.9 | 2.9 | 4.4 | 3.0 | 2.9 | ||||
| Petroselinic acid:Stearic acid | (C18:1 cis-6):(C18:0) | 2.7 | 2.3 | 5.9 | 2.9 | 4.4 | 3.0 | 2.9 | ||||
| Average extract yield (%, w/w) | 1.0 | 0.9 | 0.8 | 1.3 | 0.7 | 1.1 | 0.6 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Póvoa, O.; Farinha, N.; Lopes, V.; Machado, A.M.; Figueiredo, A.C. Coriander (Coriandrum sativum L.) from Alentejo (South Portugal)—Ethnobotany and Potential Industrial Use. Foods 2024, 13, 929. https://doi.org/10.3390/foods13060929
Póvoa O, Farinha N, Lopes V, Machado AM, Figueiredo AC. Coriander (Coriandrum sativum L.) from Alentejo (South Portugal)—Ethnobotany and Potential Industrial Use. Foods. 2024; 13(6):929. https://doi.org/10.3390/foods13060929
Chicago/Turabian StylePóvoa, Orlanda, Noémia Farinha, Violeta Lopes, Alexandra M. Machado, and Ana Cristina Figueiredo. 2024. "Coriander (Coriandrum sativum L.) from Alentejo (South Portugal)—Ethnobotany and Potential Industrial Use" Foods 13, no. 6: 929. https://doi.org/10.3390/foods13060929