Validation of a Portable Fluorescence Spectroscopy System to Monitor Heat Damage in Industrially Processed Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reception of Milk
2.2. Heat Treatments in Industrial Conditions
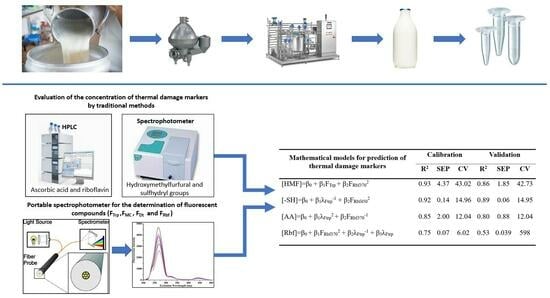
2.3. Quantification of Thermal Damage Markers
2.3.1. Hydroxymethylfurfural
2.3.2. Sulfhydryl Groups
2.3.3. Riboflavin
2.3.4. Ascorbic Acid
2.4. Fluorescence Determination
2.5. Obtaining Prediction Models Using Fluorescent Compounds
3. Results and Discussion
3.1. Analysis of Variance of Thermal Damage Markers
3.2. Prediction Models for Indicators of Thermal Damage in Milk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meshram, B.; Shakeel, A.; Suvartan, R.; Shaikh, A. Chemical markers for monitoring heat damage of processed milk: Review. Int. J. Chem. Stud. 2018, 6, 2073–2081. [Google Scholar]
- Ferrer, E.; Alegrı́a, A.; Courtois, G.; Farré, R. High-performance liquid chromatographic determination of Maillard compounds in store-brand and name-brand ultra-high-temperature-treated cows’ milk. J. Chromatogr. A 2000, 881, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Briviba, K.; Gräf, V.; Walz, E.; Guamis, B.; Butz, P. Ultra high pressure homogenization of almond milk: Physico-chemical and physiological effects. Food Chem. 2016, 192, 82–89. [Google Scholar] [CrossRef]
- Sun, D.-W.; Manzi, P.; Pizzoferrato, L. Ultrahigh Temperature Thermal Processing of milk. In Thermal Food Processing: New Technologies and Quality Issues, 2nd ed.; Sun, D.-W., Ed.; CRC Press: Hoboken, NJ, USA, 2012; p. 686. [Google Scholar]
- Van Boekel, M.A.J.S. Effect of heating on Maillard reactions in milk. Food Chem. 1998, 62, 403–414. [Google Scholar] [CrossRef]
- Morales, F.J.; Romero, C.; Jiménez-Pérez, S. An enhanced liquid-chromatographic method for 5-hydroxymethylfufural determination in UHT milk. Chromatographia 1992, 33, 45–48. [Google Scholar] [CrossRef]
- Osório, M.V.; Marques, S.S.; Oliveira, H.M.; Barreiros, L.; Segundo, M.A. Fluorometric method based on molecular recognition solid-phase extraction for determination of riboflavin in milk and infant formula. J. Food Compos. Anal. 2016, 45, 141–146. [Google Scholar] [CrossRef]
- Sharabi, S.; Okun, Z.; Shpigelman, A. Changes in the shelf life stability of riboflavin, vitamin C and antioxidant properties of milk after (ultra) high pressure homogenization: Direct and indirect effects. Innov. Food Sci. Emerg. Technol. 2018, 47, 161–169. [Google Scholar] [CrossRef]
- Zuo, R.; Zhou, S.; Zuo, Y.; Deng, Y. Determination of creatinine, uric and ascorbic acid in bovine milk and orange juice by hydrophilic interaction HPLC. Food Chem. 2015, 182, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Guingamp, M.F.; Humbert, G.; Linden, G. Determination of Sulfhydryl Groups in Milk Using Ellman’s Procedure and Clarifying Reagent®. J. Dairy. Sci. 1993, 76, 2152–2155. [Google Scholar] [CrossRef]
- Mao, X.Y.; Tong, P.S.; Gualco, S.; Vink, S. Effect of NaCl addition during diafiltration on the solubility, hydrophobicity, and disulfide bonds of 80% milk protein concentrate powder. J. Dairy. Sci. 2012, 95, 3481–3488. [Google Scholar] [CrossRef]
- Shaikh, S.; O’Donnell, C. Applications of fluorescence spectroscopy in dairy processing: A review. Curr. Opin. Food Sci. 2017, 17, 16–24. [Google Scholar] [CrossRef]
- Ayala, N.; Zamora, A.; González, C.; Saldo, J.; Castillo, M. Predicting lactulose concentration in heat-treated reconstituted skim milk powder using front-face fluorescence. Food Control 2017, 73, 110–116. [Google Scholar] [CrossRef]
- Kulmyrzaev, A.; Dufour, É. Determination of lactulose and furosine in milk using front-face fluorescence spectroscopy. Le Lait 2002, 82, 725–735. [Google Scholar] [CrossRef]
- Liu, J.; Zamora, A.; Castillo, M.; Saldo, J. Using front-face fl uorescence spectroscopy for prediction of retinol loss in milk during thermal processing. LWT Food Sci. Technol. 2018, 87, 151–157. [Google Scholar] [CrossRef]
- Kulmyrzaev, A.A.; Levieux, D.; Dufour, É. Front-face fluorescence spectroscopy allows the characterization of mild heat treatments applied to milk. Relations with the denaturation of milk proteins. J. Agric. Food Chem. 2005, 53, 502–507. [Google Scholar] [PubMed]
- Alvarado, U.; Zamora, A.; Liu, J.; Saldo, J.; Castillo, M. Rapid Quantification of Riboflavin in Milk by Front-Face Fluorescence Spectroscopy: A Preliminary Study. Foods 2020, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, U.; Zamora, A.; Arango, O.; Saldo, J.; Castillo, M. Prediction of riboflavin and ascorbic acid concentrations in skimmed heat-treated milk using front-face fluorescence spectroscopy. J. Food Eng. 2022, 318, 110869. [Google Scholar] [CrossRef]
- Keeney, M.; Bassette, R. Detection of Intermediate Compounds in the Early Stages of Browning Reaction in Milk Products. J. Dairy. Sci. 1959, 42, 945–960. [Google Scholar] [CrossRef]
- Morales, F.J.; Jiménez-Pérez, S. HMF formation during heat-treatment of milk-type products as related to milkfat content. J. Food Sci. 1999, 64, 855–859. [Google Scholar] [CrossRef]
- Albalá-Hurtado, S.; Veciana-Nogués, M.T.; Izquierdo-Pulido, M.; Mariné-Font, A. Determination of water-soluble vitamins in infant milk by high-performance liquid chromatography. J. Chromatogr. A 1997, 778, 247–253. [Google Scholar] [CrossRef]
- Romeu-Nadal, M.; Morera-Pons, S.; Castellote, A.I.; López-Sabater, M.C. Rapid high-performance liquid chromatographic method for Vitamin C determination in human milk versus an enzymatic method. J. Chromatogr. B 2006, 830, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-T. Performance evaluation of classification algorithms by k-fold and leave-one-out cross validation. Pattern Recognit. 2015, 48, 2839–2846. [Google Scholar] [CrossRef]
- Williams, P.; Sobering, D. How do we do it: A brief summary of the methods we use in developing near infrared calibrations. In Near Infrared Spectroscopy: The Future Waves; Davis, A.M.C., Williams, P., Eds.; NIR Publications: Chichester, UK, 1995. [Google Scholar]
- Morales, F.-J.; Romero, C.; Jiménez-Pérez, S. Characterization of industrial processed milk by analysis of heat-induced changes. Int. J. Food Sci. Technol. 2000, 35, 193–200. [Google Scholar] [CrossRef]
- Claeys, W.L.; Van Loey, A.M.; Hendrickx, M.E. Kinetics of hydroxymethylfurfural, lactulose and furosine formation in milk with different fat content. J. Dairy. Res. 2003, 70, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Pellegrino, L.; Resmini, P.; Luf, W. Assessment (indices) of heat treatment of milk. In Heat—Induced Changes in Milk; Fox, P.F., Ed.; International Dairy Federation: Brussels, Belgium, 1995; pp. 409–453. [Google Scholar]
- Oever, S.P.; Mayer, H.K. Analytical assessment of the intensity of heat treatment of milk and dairy products. Int. Dairy. J. 2021, 121, 105097. [Google Scholar] [CrossRef]
- Guingamp, M.F.; Humbert, G.; Midon, P.; Nicolas, M.; Linden, G. Screening procedure for evaluating heat load in commercial milks. Le Lait 1999, 79, 457–463. [Google Scholar] [CrossRef]
- Cosio, M.S.; Mannino, S.; Buratti, S. Electrochemical sensor detecting free sulfhydryl groups: Evaluation of milk heat treatment. J. Dairy. Sci. 2000, 83, 1933–1938. [Google Scholar] [CrossRef]
- Taylor, M.J.; Richardson, T. Antioxidant Activity of Skim Milk: Effect of Heat and Resultant Sulfhydryl Groups. J. Dairy. Sci. 1980, 63, 1783–1795. [Google Scholar] [CrossRef]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Techonology, 2nd ed.; Larsen and Keller Education: New York, NY, USA, 2006. [Google Scholar]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Dairy Chemistry and Biochemistry, 2nd ed.; Springer: Cork, Ireland, 2015. [Google Scholar]
- Lindmark-Månsson, H.; Fondén, R.; Pettersson, H.E. Composition of Swedish dairy milk. Int. Dairy. J. 2003, 13, 409–425. [Google Scholar] [CrossRef]
- Yasmin, A.; Huma, N.; Butt, M.S.; Zahoor, T.; Yasin, M. Seasonal variation in milk vitamin contents available for processing in Punjab, Pakistan. J. Saudi Soc. Agric. Sci. 2012, 11, 99–105. [Google Scholar] [CrossRef]
- Walstra, P.; Jenness, R. Dairy Chemistry and Physics, Ilustrada; Wiley-Blackwell: New York, NY, USA, 1984. [Google Scholar]
- Ryley, J.; Kajda, P. Vitamins in thermal processing. Food Chem. 1994, 49, 119–129. [Google Scholar] [CrossRef]
- Nohr, D.; Biesalski, H.K.; Back, E.I. Vitamins Riboflavin. In Encyclopedia of Dairy Sciences, 2nd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 704–706. [Google Scholar]
- Güneşer, O.; Karagül Yüceer, Y. Effect of ultraviolet light on water-and fat-soluble vitamins in cow and goat milk. J. Dairy. Sci. 2012, 95, 6230–6241. [Google Scholar] [CrossRef]
- Asadullah, K.-u.-N.; Tarar, O.M.; Ali, S.A.; Jamil, K.; Begum, A. Study to evaluate the impact of heat treatment on water soluble vitamins in milk. J. Pak. Med. Assoc. 2010, 60, 909–912. [Google Scholar]
- Meha, M.A. Vitamin C and riboflavin contento in Camels milk: Effects of heat treatments. Food Chem. 1994, 50, 153–155. [Google Scholar] [CrossRef]
- Sunaric, S.; Denic, M.; Kocic, G. Evaluation of riboflavin content in dairy products and non-dairy substitutes. Ital. J. Food Sci. 2012, 24, 352–358. [Google Scholar]
- Veisseyre, R. Lactología Técnica, Segunda ed.; Acribia: Zaragoza, Spain, 1988. [Google Scholar]
- Badui, S. Química de los alimentos, Cuarta ed.; Pearson Educación: Naucalpan de Juárez, Mexico, 2006. [Google Scholar]
- Alvarado, U. Aplicación de Indicadores Nativos de Fluorescencia para la Evaluación Rápida de daño Térmico en el Procesado de Leche; Universitat Aùtonoma de Barcelona: Barcelona, Spain, 2017. [Google Scholar]
| HMF (µmol·L−1) | -SH (µmol·g−1) | AA (mg·L−1) | Rbf (mg·L−1) | |
|---|---|---|---|---|
| Raw milk | 1.50 a | 1.38 a | 21.77 a | 1.33 a |
| Thermization | 1.52 a | 1.26 b | 20.27 b | 1.32 a |
| HTST pasteurization | 1.65 a | 1.21 b | 18.33 c | 1.30 a |
| HHST pasteurization | 2.84 a | 0.88 c | 16.58 d | 1.27 a |
| UHT sterilization | 9.57 b | 0.65 d | 15.48 d | 1.24 a |
| Bottle sterilization | 43.85 c | 0.12 e | 7.39 e | 1.04 b |
| Mathematical Models | Coefficients of Regression | Calibration | Validation | ||||
|---|---|---|---|---|---|---|---|
| R2 | SEC | CV | R2 | SECV | CV | ||
| β0 = 57.06 * β1 = −0.54 * | 0.92 | 4.55 | 44.80 | 0.87 | 1.51 | 44.67 |
| β0 = 93.27 * β1 = −0.68 * β2 = −1.59 × 10−9 | 0.93 | 4.37 | 43.02 | 0.86 | 1.85 | 42.73 |
| β0 = 146.9 * β1 = −0.72 * β2 = −2.50 × 10−9 * β3 = 226,061 | 0.94 | 4.14 | 40.70 | 0.87 | 2.15 | 40.42 |
| β0 = −32.85 * β1 = 11,609 * | 0.89 | 0.15 | 16.74 | 0.86 | 0.05 | 16.75 |
| β0 = −28.52 * β1 = 10,369 * β2 = −1.82 × 10−8 * | 0.92 | 0.14 | 14.96 | 0.89 | 0.06 | 14.95 |
| β0 = −31.8 β1 = 10,937 β2 = −1.82 × 10−8 * β3 = 4.7 × 10−3 | 0.92 | 0.04 | 15.29 | 0.88 | 0.07 | 15.52 |
| β0 = 194.32 * β1 = −0.001 * | 0.84 | 2.03 | 12.22 | 0.81 | 0.70 | 12.22 |
| β0 = 230.94 * β1 = −0.002 * β2 = −1,319,764 | 0.85 | 2.00 | 12.04 | 0.80 | 0.88 | 12.04 |
| β0 = 190.71 * β1 = −0.001 * β2 = −2,555,009 * β3 = −211.42 * | 0.90 | 1.76 | 10.56 | 0.80 | 0.94 | 10.52 |
| β0 = 1.89 * β1 = −4.19 × 10−11 * | 0.65 | 0.08 | 6.73 | 0.54 | 0.028 | 6.70 |
| β0 = −2.27 * β1 = −2.71 × 10−11 * β2 = 1352.5 | 0.69 | 0.08 | 6.48 | 0.53 | 0.035 | 6.46 |
| β0 = −475.71 * β1 = −4.21 × 10−11 * β2 = 82,747 β3 = 0.69 | 0.75 | 0.07 | 6.02 | 0.53 | 0.039 | 5.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado, U.; Zamora, A.; Arango, O.; Saldo, J.; Castillo, M. Validation of a Portable Fluorescence Spectroscopy System to Monitor Heat Damage in Industrially Processed Milk. Foods 2024, 13, 780. https://doi.org/10.3390/foods13050780
Alvarado U, Zamora A, Arango O, Saldo J, Castillo M. Validation of a Portable Fluorescence Spectroscopy System to Monitor Heat Damage in Industrially Processed Milk. Foods. 2024; 13(5):780. https://doi.org/10.3390/foods13050780
Chicago/Turabian StyleAlvarado, Ulises, Anna Zamora, Oscar Arango, Jordi Saldo, and Manuel Castillo. 2024. "Validation of a Portable Fluorescence Spectroscopy System to Monitor Heat Damage in Industrially Processed Milk" Foods 13, no. 5: 780. https://doi.org/10.3390/foods13050780