Impact of Corn Starch Molecular Structures on Texture, Water Dynamics, Microstructure, and Protein Structure in Silver Carp (Hypophthalmichthys molitrix) Surimi Gel
Abstract
:1. Introduction
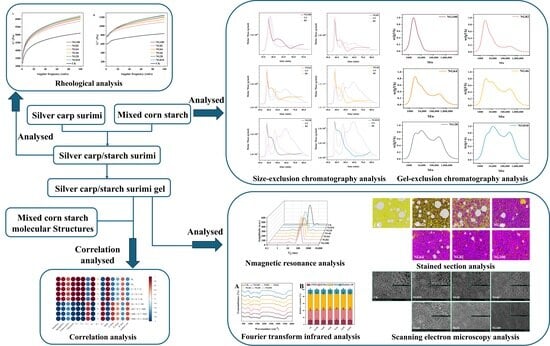
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Molecular Weight Distribution
2.4. Chain-Length Distributions (CLDs) of Debranched Starch
2.5. Amylose Content, Amylopectin Content, and AM/AP of Starch
2.6. Dynamic Rheological Measurement
2.7. Textural Profile Analysis (TPA)
2.8. Water-Holding Capacity (WHC)
2.9. LF-NMR Spin-Spin Relaxation (T2) Measurement
2.10. Distribution and Morphology of Gels
2.11. Cryo-Scanning Electron Microscopy (Cryo-SEM)
2.12. Fourier Transform Infrared (FT-IR) Spectroscopy
2.13. Statistical Analysis
3. Results
3.1. Molecular Structure of Starch
3.2. Rheological Properties of the Surimi-Starch System
3.3. Textural Properties of the Surimi Gel
3.4. Water-Holding Capacity of the Surimi Gel
3.5. LF-NMR Analysis of the Surimi Gel
3.6. Starch Distribution by Light Microscopy
3.7. Microstructure by Cryo-SEM
3.8. Protein Secondary Structure
3.9. Relationships between Structure and Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alipour, H.J.; Rezaei, M.; Shabanpour, B.; Tabarsa, M. Effects of sulfated polysaccharides from green alga Ulva intestinalis on physicochemical properties and microstructure of silver carp surimi. Food Hydrocoll. 2018, 74, 87–96. [Google Scholar] [CrossRef]
- Sun, F.; Huang, Q.; Hu, T.; Xiong, S.; Zhao, S. Effects and mechanism of modified starches on the gel properties of myofibrillar protein from grass carp. Int. J. Biol. Macromol. 2014, 64, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kok, N.; Thawornchinsombut, S.; Park, J.W. Manufacture of surimi. In Surimi and Surimi Seafood; CRC Press: Boca Raton, FL, USA, 2014; pp. 285–300. [Google Scholar]
- Kong, W.; Zhang, T.; Feng, D.; Xue, Y.; Wang, Y.; Li, Z.; Yang, W.; Xue, C. Effects of modified starches on the gel properties of Alaska Pollock surimi subjected to different temperature treatments. Food Hydrocoll. 2016, 56, 20–28. [Google Scholar] [CrossRef]
- Liu, H.; Nie, Y.; Chen, H. Effect of different starches on colors and textural properties of surimi-starch gels. Int. J. Food Prop. 2014, 17, 1439–1448. [Google Scholar] [CrossRef]
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Effect of small granules in potato starch and wheat starch on quality changes of direct heated surimi gels after freezing. Food Hydrocoll. 2020, 104, 105732. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, C.M. Effect of starch on textural properties of surimi gel. J. Food Sci. 1987, 52, 722–725. [Google Scholar] [CrossRef]
- Tappiban, P.; Sraphet, S.; Srisawad, N.; Wu, P.; Han, H.; Smith, D.R.; Bao, J.; Triwitayakorn, K. Effects of cassava variety and growth location on starch fine structure and physicochemical properties. Food Hydrocoll. 2020, 108, 106074. [Google Scholar] [CrossRef]
- Kong, X.; Bertoft, E.; Bao, J.; Corke, H. Molecular structure of amylopectin from amaranth starch and its effect on physico-chemical properties. Int. J. Biol. Macromol. 2008, 43, 377–382. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Rheological properties in relation to molecular structure of quinoa starch. Int. J. Biol. Macromol. 2018, 114, 767–775. [Google Scholar] [CrossRef]
- Du, J.; Pan, R.; Obadi, M.; Li, H.; Shao, F.; Sun, J.; Wang, Y.; Qi, Y.; Xu, B. In vitro starch digestibility of buckwheat cultivars in comparison to wheat: The key role of starch molecular structure. Food Chem. 2022, 368, 130806. [Google Scholar] [CrossRef]
- Tao, K.; Yu, W.; Prakash, S.; Gilbert, R.G. High-amylose rice: Starch molecular structural features controlling cooked rice texture and preference. Carbohyd. Polym. 2019, 219, 251–260. [Google Scholar] [CrossRef]
- Ekonomou, S.Ι.; Hadnađev, M.; Gioxari, A.; Abosede, O.R.; Soe, S.; Stratakos, A.C. Advancing dysphagia-oriented multi-ingredient meal development: Optimising hydrocolloid incorporation in 3D printed nutritious meals. Food Hydrocoll. 2024, 147, 109300. [Google Scholar] [CrossRef]
- Cortez-Trejo, M.C.; Gaytán-Martínez, M.; Reyes-Vega, M.L.; Mendoza, S. Protein-gum-based gels: Effect of gum addition on microstructure, rheological properties, and water retention capacity. Trends Food Sci. Technol. 2021, 116, 303–317. [Google Scholar] [CrossRef]
- Kang, X.; Jia, S.; Gao, W.; Wang, B.; Zhang, X.; Tian, Y.; Sun, Q.; Atef, M.; Cui, B.; Abd El-Aty, A.M. The formation of starch-lipid complexes by microwave heating. Food Chem. 2022, 382, 132319. [Google Scholar] [CrossRef]
- Pan, L.; Chen, F.; Yang, Y.; Li, Q.; Fan, X.; Zhao, D.; Liu, Q.; Zhang, C. The underlying starch structures of rice grains with different digestibilities but similarly high amylose contents. Food Chem. 2022, 379, 132071. [Google Scholar] [CrossRef]
- Hanashiro, I.; Abe, J.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohyd. Res. 1996, 283, 151–159. [Google Scholar] [CrossRef]
- GB/T 15683-2008; Rice-Determination of Amylose Content. Chinese National Standard. Available online: http://www.foodmate.net (accessed on 16 January 2024).
- Luo, H.; Guo, C.; Lin, L.; Si, Y.; Gao, X.; Xu, D.; Jia, R.; Yang, W. Combined use of rheology, LF-NMR, and MRI for characterizing the gel properties of hairtail surimi with potato starch. Food Bioprocess Technol. 2020, 13, 637–647. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, G.; Li, J.; Tian, F.; Zheng, B.; Gao, P.; Zhou, R. Comparative study on the effect of different salts on surimi gelation and gel properties. Food Hydrocoll. 2023, 144, 108982. [Google Scholar] [CrossRef]
- Piao, X.; Huang, J.; Sun, Y.; Zhao, Y.; Zheng, B.; Zhou, Y.; Yu, H.; Zhou, R.; Cullen, P.J. Inulin for surimi gel fortification: Performance and molecular weight-dependent effects. Carbohyd. Polym. 2023, 305, 120550. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lv, Y.; Jia, H.; Mráz, J.; Gu, Y.; Xu, X.; Li, S.; Dong, X.; Pan, J. Improvement of flavor and gel properties of silver carp surimi product by Litsea cubeba oil high internal phase emulsions. LWT-Food Sci. 2024, 192, 115745. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Zeng, L.; Huang, Q.; Zhang, J.; Wen, X.; Xiong, S.; Yin, T.; Zhang, B. Effects of oil-modified crosslinked/acetylated starches on silver carp surimi gel: Texture properties, water mobility, microstructure, and related mechanisms. Food Res. Int. 2022, 158, 111521. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, S.; Zhao, D.; Zhang, J.; Gu, S.; Pan, Z.; Ding, Y. Changes in physicochemical properties and protein structure of surimi enhanced with camellia tea oil. LWT-Food Sci. Technol. 2017, 84, 562–571. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Q.; Xiao, N.; Du, Y.; Feng, Q.; Shi, W. Changes in Gel Structure and Chemical Interactions of Hypophthalmichthys molitrix Surimi Gels: Effect of Setting Process and Different Starch Addition. Foods 2021, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gong, B. Relations between rice starch fine molecular and lamellar/crystalline structures. Food Chem. 2021, 353, 129467. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Capitani, T.; Trzasko, P.; Jeffcoat, R. Molecular structure of a low-amylopectin starch and other high-amylose maize starches. J. Cereal Sci. 1998, 27, 289–299. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Bhandari, B.; Liu, Y. Investigation on lemon juice gel as food material for 3D printing and optimization of printing parameters. LWT-Food Sci. Technol. 2018, 87, 67–76. [Google Scholar] [CrossRef]
- Alakhrash, F.; Anyanwu, U.; Tahergorabi, R. Physicochemical properties of Alaska pollock (Theragra chalcograma) surimi gels with oat bran. LWT-Food Sci. Technol. 2016, 66, 41–47. [Google Scholar] [CrossRef]
- Mi, H.; Wang, C.; Su, Q.; Li, X.; Yi, S.; Li, J. The effect of modified starches on the gel properties and protein conformation of Nemipterus virgatus surimi. J. Texture Stud. 2019, 50, 571–581. [Google Scholar] [CrossRef]
- Friedman, H.H.; Whitney, J.E.; Szczesniak, A.S. The texturometer-A new instrument for objective texture measurement. J. Food Sci. 1963, 28, 390–396. [Google Scholar] [CrossRef]
- Wu, W.; Que, F.; Li, X.; Shi, L.; Deng, W.; Fu, X.; Xiong, G.; Sun, J.; Wang, L.; Xiong, S. Effects of enzymatic konjac glucomannan hydrolysates on textural properties, microstructure, and water distribution of grass carp surimi gels. Foods 2022, 11, 750. [Google Scholar] [CrossRef]
- Mi, H.; Su, Q.; Chen, J.; Yi, S.; Li, X.; Li, J. Starch-fatty acid complexes improve the gel properties and enhance the fatty acid content of Nemipterus virgatus surimi under high-temperature treatment. Food Chem. 2021, 362, 130253. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Observations on the impact of amylopectin and amylose structure on the swelling of starch granules. Food Hydrocoll. 2020, 103, 105663. [Google Scholar] [CrossRef]
- Pereira, J.; Malairaj, S.; Brohi, S.A.; Boateng, E.F.; Zhang, W. Impact of unripe banana flour on water states, rheological behaviour and structural properties of myofibrillar protein composite gel. LWT-Food Sci. Technol. 2020, 125, 109276. [Google Scholar] [CrossRef]
- Dong, M.; Tian, H.; Xu, Y.; Han, M. Effects of pulsed electric fields on the conformation and gelation properties of myofibrillar proteins isolated from pale, soft, exudative (PSE)-like chicken breast meat: A molecular dynamics study. Food Chem. 2020, 342, 128306. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Li, X.; Zeng, L.; Huang, J.; Huang, Q.; Zhang, B. Effect of oil modification on the multiscale structure and gelatinization properties of crosslinked starch and their relationship with the texture and microstructure of surimi/starch composite gels. Food Chem. 2022, 391, 133236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, Y.; Xu, J.; Li, Z. Effects of high-temperature treatment (≥100 °C) on Alaska Pollock (Theragrachalcogramma) surimi gels. J. Food Eng. 2013, 115, 115–120. [Google Scholar] [CrossRef]
- Wootton, M.; Bamunuarachchi, A. Water binding capacity of commercial produced native and modified starches. Starch-Stärke 1978, 30, 306–309. [Google Scholar] [CrossRef]
- Ghazal, A.F.; Zhang, M.; Bhandari, B.; Chen, H. Investigation on spontaneous 4D changes in color and flavor of healthy 3D printed food materials over time in response to external or internal pH stimulus. Food Res. Int. 2021, 142, 110215. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Yoo, S.H.; Lim, S.T. Water effect on the interaction between amylose and amylopectin during retrogradation. Carbohyd. Polym. 2011, 86, 1671–1674. [Google Scholar] [CrossRef]
- Chen, D.; Fang, F.; Federici, E.; Campanell, O. Rheology, microstructure and phase behavior of potato starch-protein fibril mixed gel. Carbohyd. Polym. 2020, 239, 116247. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Ai, Y. Gelation mechanisms of granular and non-granular starches with variations in molecular structures. Food Hydrocoll. 2022, 129, 107658. [Google Scholar] [CrossRef]
- Mi, H.; Li, Y.; Wang, C.; Yi, S. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Yang, H.; Park, J.W. Effects of starch properties and thermal-processing conditions on surimi–starch gels. LWT-Food Sci. Technol. 1998, 31, 344–353. [Google Scholar] [CrossRef]
- Wu, M.; Wang, G.; Ge, Q.; Yu, H. Rheology and microstructure of myofibrillar protein–starch composite gels: Comparison of native and modified starches. Int. J. Biol. Macromol. 2018, 118, 988–996. [Google Scholar] [CrossRef]
- Fan, M.; Huang, Q.; Zhong, S.; Li, X.; Xiong, S.; Xie, J.; Yin, T.; Zhang, B.; Zhao, S. Gel properties of myofibrillar protein as affected by gelatinization and retrogradation behaviors of modified starches with different crosslinking and acetylation degrees. Food Hydrocoll. 2019, 96, 604–616. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhou, Y.; Ma, F. Effect of resistant corn starch on the thermal gelling properties of chicken breast myosin. Food Hydrocoll. 2019, 96, 681–687. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Wang, H.; Ye, Q. Effects of a highly resistant rice starch and pre-incubation temperatures on the physicochemical properties of surimi gel from grass carp (Ctenopharyn Odon Idellus). Food Chem. 2014, 145, 212–219. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Z.; Pan, Y.; Jiang, P.; Pan, J.; Yu, C.; Dong, X. Effect of κ-carrageenan on quality improvement of 3D printed Hypophthalmichthys molitrix-sea cucumber compound surimi product. LWT-Food Sci. Technol. 2022, 154, 112279. [Google Scholar] [CrossRef]
- Zhuang, X.; Jiang, X.; Zhou, H.; Chen, Y.; Zhao, Y.; Yang, H.; Zhou, G. Insight into the mechanism of physicochemical influence by three polysaccharides on myofibrillar protein gelation. Carbohyd. Polym. 2020, 229, 115449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Shi, J.; Zhu, B.; Luo, Y. Changes in chemical interactions and gel properties of heat-induced surimi gels from silver carp (Hypophthalmichthys molitrix) fillets during setting and heating: Effects of different washing solutions. Food Hydrocoll. 2018, 75, 116–124. [Google Scholar] [CrossRef]
- Park, J.W.; Lin, T.-M.; Yongsawatdigul, J. New developments in manufacturing of surimi and surimi seafood. Food Rev. Int. 1997, 13, 577–610. [Google Scholar] [CrossRef]
- Yang, X.; Pan, Y.; Li, S.; Li, C.; Li, E. Effects of amylose and amylopectin molecular structures on rheological, thermal and textural properties of soft cake batters. Food Hydrocoll. 2022, 133, 107980. [Google Scholar] [CrossRef]
- Petcharat, T.; Benjakul, S. Effect of gellan incorporation on gel properties of bigeye snapper surimi. Food Hydrocoll. 2018, 77, 746–753. [Google Scholar] [CrossRef]








| Samples | Structure Parameters | ||||||||||
| Mw | Mp | Amylose | Amylopectin | AM/AP | |||||||
| NG100 | 65,304.72 ± 1704.51 a | 113,771.44 ± 9508.12 a | 6.44 ± 0.37 f | 93.56 ± 0.37 a | 0.07 ± 0.00 f | ||||||
| NG82 | 52,146.74 ± 1642.80 b | 93,568.84 ± 2904.67 b | 19.55 ± 0.76 e | 80.45 ± 0.76 b | 0.24 ± 0.01 e | ||||||
| NG64 | 39,400.75 ± 1452.86 c | 71,146.25 ± 3697.08 c | 32.34 ± 1.72 d | 67.66 ± 1.72 c | 0.48 ± 0.04 d | ||||||
| NG46 | 26,794.76 ± 686.66 d | 47,966.99 ± 1300.05 d | 45.25 ± 2.60 c | 54.75 ± 2.60 d | 0.83 ± 0.09 c | ||||||
| NG28 | 14,578.11 ± 467.40 e | 24,154.40 ± 1127.36 e | 58.73 ± 1.16 b | 41.27 ± 1.16 e | 1.42 ± 0.07 b | ||||||
| NG010 | 1893.79 ± 73.25 f | 1331.81 ± 102.23 f | 69.89 ± 1.31 a | 30.11 ± 1.31 f | 2.33 ± 0.14 a | ||||||
| Samples | DP | ||||||||||
| 6 < X ≤ 12 (A) | 12 < X ≤ 24 (B1) | 24 < X ≤ 36 (B2) | 36 < X ≤ 100 (B3) | 100 < X ≤ 1000 | 1000 < X ≤ 5000 | 5000 < X ≤ 20,000 | |||||
| NG100 | 28.42 ± 2.16 a | 33.94 ± 2.58 a | 14.53 ± 0.70 a | 16.29 ± 0.44 d | 1.26 ± 0.20 f | 0.17 ± 0.02 f | 0.25 ± 0.01 f | ||||
| NG82 | 23.49 ± 1.51 b | 29.04 ± 1.55 b | 12.94 ± 0.25 b | 16.96 ± 0.09 cd | 8.15 ± 0.25 e | 4.45 ± 0.24 e | 1.07 ± 0.07 e | ||||
| NG64 | 18.61 ± 1.81 c | 23.79 ± 0.91 c | 11.34 ± 0.23 c | 17.55 ± 1.18 bcd | 14.56 ± 1.72 d | 8.72 ± 0.33 d | 1.85 ± 0.22 d | ||||
| NG46 | 13.91 ± 0.96 d | 18.56 ± 1.06 d | 9.71 ± 0.11 d | 18.02 ± 0.74 abc | 21.84 ± 0.42 c | 12.95 ± 0.26 c | 2.72 ± 0.04 c | ||||
| NG28 | 9.01 ± 0.13 e | 13.40 ± 0.68 e | 8.10 ± 0.10 e | 18.77 ± 0.59 ab | 28.42 ± 1.76 b | 17.25 ± 0.44 b | 3.52 ± 0.03 b | ||||
| NG010 | 4.19 ± 0.36 f | 8.32 ± 0.42 f | 6.48 ± 0.02 f | 19.30 ± 1.03 a | 35.25 ± 2.15 a | 21.54 ± 0.84 a | 4.36 ± 0.11 a | ||||
| Sample | Texture Analysis | |||
|---|---|---|---|---|
| Hardness (g) | Springiness | Cohesiveness | Chewiness | |
| CK | 1028.54 ± 11.75 g | 0.85 ± 0.00 e | 0.70 ± 0.00 a | 615.33 ± 8.38 g |
| NG100 | 2357.44 ± 16.62 b | 0.90 ± 0.01 b | 0.68 ± 0.01 c | 1439.05 ± 15.62 b |
| NG82 | 2438.10 ± 35.28 a | 0.92 ± 0.00 a | 0.69 ± 0.00 b | 1559.55 ± 23.15 a |
| NG64 | 2105.61 ± 21.20 c | 0.90 ± 0.00 b | 0.68 ± 0.00 c | 1267.58 ± 28.23 c |
| NG46 | 1933.97 ± 34.39 d | 0.89 ± 0.01 c | 0.68 ± 0.00 c | 1145.99 ± 5.31 d |
| NG28 | 1759.47 ± 9.52 e | 0.87 ± 0.00 d | 0.68 ± 0.00 cd | 1051.32 ± 3.77 e |
| NG010 | 1534.18 ± 18.65 f | 0.85 ± 0.00 e | 0.67 ± 0.00 d | 862.51 ± 4.76 f |
| Sample | WHC (%) | T2 (ms) | A2 (%) | ||||
|---|---|---|---|---|---|---|---|
| T21 | T22 | T23 | A21 | A22 | A23 | ||
| CK | 69.50 ± 0.44 e | 1.79 ± 0.15 f | 123.22 ± 4.88 a | 822.75 ± 57.09 a | 0.60 ± 0.03 a | 97.22 ± 0.28 a | 2.66 ± 0.11 b |
| NG100 | 93.16 ± 0.06 a | 10.37 ± 0.72 a | 120.41 ± 4.88 a | - | 5.75 ± 0.06 b | 94.15 ± 0.13 c | - |
| NG82 | 92.89 ± 0.18 a | 9.46 ± 0.77 b | 109.70 ± 0.00 b | - | 5.42 ± 0.09 c | 94.48 ± 0.10 c | - |
| NG64 | 90.12 ± 0.21 b | 8.42 ± 0.59 c | 100.05 ± 3.96 c | - | 4.77 ± 0.12 d | 95.08 ± 0.08 b | - |
| NG46 | 90.07 ± 0.26 b | 5.55 ± 0.39 d | 83.23 ± 5.77 d | 682.97 ± 27.68 b | 3.94 ± 0.08 e | 94.97 ± 0.21 b | 0.80 ± 0.04 d |
| NG28 | 86.14 ± 0.33 c | 4.21 ± 0.29 e | 67.59 ± 4.69 e | 517.37 ± 20.98 c | 2.54 ± 0.11 f | 94.94 ± 0.08 b | 2.38 ± 0.11 c |
| NG010 | 78.86 ± 1.16 d | 2.59 ± 0.18 f | 54.88 ± 3.81 f | 450.30 ± 18.26 d | 1.62 ± 0.07 g | 93.23 ± 0.33 d | 4.64 ± 0.11 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Yang, X.; Lin, S.; Yang, Y.; Yu, J.; Du, X.; Tang, Y. Impact of Corn Starch Molecular Structures on Texture, Water Dynamics, Microstructure, and Protein Structure in Silver Carp (Hypophthalmichthys molitrix) Surimi Gel. Foods 2024, 13, 675. https://doi.org/10.3390/foods13050675
Jiang C, Yang X, Lin S, Yang Y, Yu J, Du X, Tang Y. Impact of Corn Starch Molecular Structures on Texture, Water Dynamics, Microstructure, and Protein Structure in Silver Carp (Hypophthalmichthys molitrix) Surimi Gel. Foods. 2024; 13(5):675. https://doi.org/10.3390/foods13050675
Chicago/Turabian StyleJiang, Congyun, Xin Yang, Songyi Lin, Yumeng Yang, Jinzhi Yu, Xinqi Du, and Yue Tang. 2024. "Impact of Corn Starch Molecular Structures on Texture, Water Dynamics, Microstructure, and Protein Structure in Silver Carp (Hypophthalmichthys molitrix) Surimi Gel" Foods 13, no. 5: 675. https://doi.org/10.3390/foods13050675