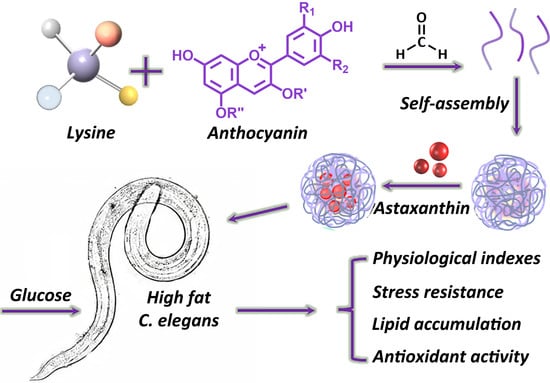
Lipid-Lowering and Antioxidant Effects of Self-Assembled Astaxanthin–Anthocyanin Nanoparticles on High-Fat Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AXT-ACN NPs
2.3. Caenorhabditis Elegans Culture and Synchronization
2.4. Lifespan Assay
2.5. Reproduction Assay
2.6. Head Swing Assay
2.7. Stress Resistance Assay
2.8. Oil Red O Staining of C. elegans
2.9. Measurement of Lipofuscin Accumulation
2.10. Determination of the Reactive Oxygen Species (ROS) Level
2.11. Measurement of TG, NEFA, GSH-PX, SOD, CAT, and MDA Levels
2.12. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Hu, F.B. Sugar-Sweetened Beverages, Obesity, Type 2 Diabetes Mellitus, and Cardiovascular Disease Risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef]
- Savova, M.S.; Todorova, M.N.; Apostolov, A.G.; Yahubyan, G.T.; Georgiev, M.I. Betulinic acid counteracts the lipid accumulation in Caenorhabditis elegans by modulation of nhr-49 expression. Biomed. Pharmacother. 2022, 156, 12. [Google Scholar] [CrossRef]
- Breakthrough, F.E. Innovation Focus in 2022. Available online: https://www.cell.com/the-innovation/fulltext/S2666-6758(22)00167-9?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2666675822001679%3Fshowall%3Dtrue (accessed on 30 January 2024).
- Novelli, G.; Cassadonte, C.; Sbraccia, P.; Biancolella, M. Genetics: A Starting Point for the Prevention and the Treatment of Obesity. Nutrients 2023, 15, 2782. [Google Scholar] [CrossRef]
- Xie, J.T.; Hou, X.N.; He, W.S.; Xiao, J.; Cao, Y.; Liu, X.J. Astaxanthin reduces fat storage in a fat-6 fat-7 dependent manner determined using high fat Caenorhabditis elegans. Food Funct. 2023, 14, 7347–7360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zuo, T.; Wan, Y.; Xu, Z.; Cheung, C.; Li, A.Y.; Zhu, W.; Tang, W.; Chan, P.K.S.; Chan, F.K.L.; et al. Multi-omic analyses identify mucosa bacteria and fecal metabolites associated with weight loss after fecal microbiota transplantation. Innovation 2022, 3, 100304. [Google Scholar] [CrossRef]
- Tu, H.; McQuade, J.L.; Davies, M.A.; Huang, M.; Xie, K.; Ye, Y.; Chow, W.-H.; Rodriguez, A.; Wu, X. Body mass index and survival after cancer diagnosis: A pan-cancer cohort study of 114 430 patients with cancer. Innovation 2022, 3, 100344. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Goulis, D.G.; Giouleme, O.; Germanidis, G.S.; Goulas, A. Anti-obesity Medications for the Management of Nonalcoholic Fatty Liver Disease. Curr. Obes. Rep. 2022, 11, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Fang, Z.; Ge, J.; Li, H. Nanotechnology for cardiovascular diseases. Innovation 2022, 3, 100214. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Shimomura, I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord. 2014, 15, 1–10. [Google Scholar] [CrossRef]
- Radice, R.P.; Limongi, A.R.; Viviano, E.; Padula, M.C.; Martelli, G.; Bermano, G. Effects of astaxanthin in animal models of obesity-associated diseases: A systematic review and meta-analysis. Free Radic. Biol. Med. 2021, 171, 156–168. [Google Scholar] [CrossRef]
- Wang, M.; Ma, H.T.; Guan, S.Y.; Luo, T.; Zhao, C.C.; Cai, G.P.; Zheng, Y.B.; Jia, X.Y.; Di, J.B.; Li, R.Z.; et al. Astaxanthin from Haematococcus pluvialis alleviates obesity by modulating lipid metabolism and gut microbiota in mice fed a high-fat diet. Food Funct. 2021, 12, 9719–9738. [Google Scholar] [CrossRef]
- Wang, J.H.; Liu, S.W.; Wang, H.; Xiao, S.; Li, C.; Li, Y.; Liu, B.N. Xanthophyllomyces dendrorhous-Derived Astaxanthin Regulates Lipid Metabolism and Gut Microbiota in Obese Mice Induced by A High-Fat Diet. Mar. Drugs 2019, 17, 337. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive Effects of Astaxanthin on Lipid Profiles and Oxidative Stress in Overweight Subjects. Plant Food Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Calvo, M.M.; Gómez-Estaca, J. Recent Advances in Astaxanthin Micro/Nanoencapsulation to Improve Its Stability and Functionality as a Food Ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef]
- Guo, M.; Cui, W.N.; Li, Y.C.; Fei, S.Y.; Sun, C.F.; Tan, M.Q.; Su, W.T. Microfluidic fabrication of size-controlled nanocarriers with improved stability and biocompatibility for astaxanthin delivery. Food Res. Int. 2023, 170, 12. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Su, H.M.; Sun, C.D.; Zheng, X.D.; Chen, W. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci. Technol. 2018, 72, 13–24. [Google Scholar] [CrossRef]
- Wang, Y.L.; Sun, Y.D.; Wang, X.G.; Wang, Y.; Liao, L.X.; Zhang, Y.H.; Fang, B.S.; Fu, Y.S. Novel antioxidant peptides from Yak bones collagen enhanced the capacities of antiaging and antioxidant in Caenorhabditis elegans. J. Funct. Food. 2022, 89, 8. [Google Scholar] [CrossRef]
- Chen, K.X.; Shi, L.F.; Ren, Z.Y.; Weng, W.Y. Antioxidant characteristics of hydrolysate from low-value sea cucumber: In vitro and in vivo activities of Caenorhabditis elegans. Food Chem. X 2023, 19, 100836. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, X.D.; Li, J.X.; Li, Y.; Zhou, C.F.; Xiang, S.Y.; Tan, M.Q. Preparation and evaluation of ovalbumin-fucoidan nanoparticles for nicotinamide mononucleotide encapsulation with enhanced stability and anti-aging activity. Food Chem. 2023, 418, 10. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, Z.G.; Guo, Y.J.; Lu, S.; Du, H.Z.; Cao, Y. Antioxidant capacity of flavonoids from Folium Artemisiae Argyi and the molecular mechanism in Caenorhabditis elegans. J. Ethnopharmacol. 2021, 279, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Gao, L.E.; Huang, W.K.; Ma, Y.; Muhammad, I.; Hanif, A.; Ding, Z.T.; Guo, X.S. Antioxidant properties of lactic acid bacteria isolated from traditional fermented yak milk and their probiotic effects on the oxidative senescence of Caenorhabditis elegans. Food Funct. 2022, 13, 3690–3703. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, W.; Tang, Y.Z.; Lin, C.X.; Cao, Y.; Chen, Y.J. Mechanism of Pentagalloyl Glucose in Alleviating Fat Accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2019, 67, 14110–14120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.H.; Yang, X.; Xu, W.; Cai, X.B.; Wang, M.X.; Xu, Y.Y.; Yu, P.L.; Zhang, J.; Zheng, Y.F.; Chen, J.; et al. Evaluation of the effects of three sulfa sweeteners on the lifespan and intestinal fat deposition in C. elegans. Food Res. Int. 2019, 122, 66–76. [Google Scholar] [CrossRef]
- Sun, J.; Zhong, X.Y.; Sun, D.D.; Xu, L.R.; Shi, L.L.; Sui, J.L.; Liu, Y.J. Anti-aging effects of polysaccharides from ginseng extract residues in Caenorhabditis elegans. Int. J. Biol. Macromol. 2023, 225, 1072–1084. [Google Scholar] [CrossRef]
- Li, J.; Wuliji, O.; Li, W.; Jiang, Z.-G.; Ghanbari, H.A. Oxidative Stress and Neurodegenerative Disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef]
- Franco-Juárez, B.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Cárdenas-Rodríguez, N.; Arreguin-Espinosa, R.; de la Cruz, V.P.; Ortega-Cuellar, D. Effects of High Dietary Carbohydrate and Lipid Intake on the Lifespan of C. elegans. Cells 2021, 10, 2359. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.B.; Wang, P.; Dong, C.; Zhang, J.; Wang, X.; Pei, H.F. Oxidative Stress Signaling Mediated Pathogenesis of Diabetic Cardiomyopathy. Oxidative Med. Cell. Longev. 2022, 2022, 8. [Google Scholar] [CrossRef]
- Pandey, R.; Mani, D.; Shanker, K.; Bawankule, D.U.; Chanda, D.; Lal, R.K.; Pal, A.; Khare, P.; Kumar, N.; Tandon, S. Towards the development of phytoextract based healthy ageing cognitive booster formulation, explored through Caenorhabditis elegans model. Nucleus 2022, 65, 303–320. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.T.; Lin, X.L.; Zheng, X.Q.; Qi, H.M.; Chen, J.P.; Zeng, X.F.; Bai, W.D.; Xiao, G.S. Biological Activities and Solubilization Methodologies of Naringin. Foods 2023, 12, 2327. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Yang, Z.-T.; Mao, E.-Q.; Chen, Y.; Chen, E.-Z. Free fatty acids-induced neutrophil extracellular traps lead to dendritic cells activation and T cell differentiation in acute lung injury. Aging 2021, 13, 26148–26160. [Google Scholar] [CrossRef]
- Wang, Q.R.; Wang, J.; Li, N.N.; Liu, J.Y.; Zhou, J.N.; Zhuang, P.W.; Chen, H.X. A Systematic Review of Orthosiphon stamineus Benth. in the Treatment of Diabetes and Its Complications. Molecules 2022, 27, 444. [Google Scholar] [CrossRef]
- Andrade, A.W.L.; Guerra, G.C.B.; Araújo, D.F.D.; de Araujo, R.F.; de Araújo, A.A.; de Carvalho, T.G.; Fernandes, J.M.; Diez-Echave, P.; Hidalgo-García, L.; Rodriguez-Cabezas, M.E.; et al. Anti-Inflammatory and Chemopreventive Effects of Bryophyllum pinnatum (Lamarck) Leaf Extract in Experimental Colitis Models in Rodents. Front. Pharmacol. 2020, 11, 18. [Google Scholar] [CrossRef]
- Roh, H.T.; So, W.Y. The effects of aerobic exercise training on oxidant-antioxidant balance, neurotrophic factor levels, and blood-brain barrier function in obese and non-obese men. J. Sport Health Sci. 2017, 6, 447–453. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Shimomura, I.; Nakamura, T.; Keno, Y.; Kotani, K.; Tokunaga, K. Pathophysiology and pathogenesis of visceral fat obesity. Obes. Res. 1995, 3, 187s–194s. [Google Scholar] [CrossRef]
- Doi, H.; Hoshino, Y.; Nakase, K.; Usuda, Y. Reduction of hydrogen peroxide stress derived from fatty acid beta-oxidation improves fatty acid utilization in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 629–639. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Tian, Y.; Zhou, F.; Ma, J.; Xia, S.; Yang, T.; Ma, L.; Zeng, Q.; Liu, G.; et al. Obese Ningxiang pig-derived microbiota rewires carnitine metabolism to promote muscle fatty acid deposition in lean DLY pigs. Innovation 2023, 4, 100486. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Fu, X.Q.; Chen, J.; Ma, J.F. Application of Caenorhabditis elegans in Lipid Metabolism Research. Int. J. Mol. Sci. 2023, 24, 1173. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.C.; Yue, Y.R.; Shen, P.Y.; Yang, J.J.; Park, Y. Cranberry Product Decreases Fat Accumulation in Caenorhabditis elegans. J. Med. Food 2016, 19, 427–433. [Google Scholar] [CrossRef]
- Yang, H.; Su, J.L.; Qi, J. Autotoxicity effect of water extracts from rhizosphere soil of Elymus sibiricus in different planting years on seed germination, physiological characteristics and phytohormones of seedlings. PeerJ 2022, 10, e13768. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Wang, Y.; Oberley, L.W.; Murhammer, D.W. Antioxidant defense systems of two lipidopteran insect cell lines. Free Radic. Biol. Med. 2001, 30, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Y.; Chen, Y.T.; Wang, G.; Feng, T.; Shen, M.; Xiao, B.; Gu, J.Y.; Wang, W.M.; Li, J.; Zhang, Y.J. Evaluation of the antioxidant effects of acid hydrolysates from Auricularia auricular polysaccharides using a Caenorhabditis elegans model. Food Funct. 2019, 10, 5531–5543. [Google Scholar] [CrossRef] [PubMed]
- Li, R.D.; Zhang, P.; Li, C.G.; Yang, W.C.; Yin, Y.P.; Tao, K.X. Tert-butylhydroquinone mitigates Carbon Tetrachloride induced Hepatic Injury in mice. Int. J. Med. Sci. 2020, 17, 2095–2103. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Guo, M.; Tan, M.; Su, W. Lipid-Lowering and Antioxidant Effects of Self-Assembled Astaxanthin–Anthocyanin Nanoparticles on High-Fat Caenorhabditis elegans. Foods 2024, 13, 514. https://doi.org/10.3390/foods13040514
Yu D, Guo M, Tan M, Su W. Lipid-Lowering and Antioxidant Effects of Self-Assembled Astaxanthin–Anthocyanin Nanoparticles on High-Fat Caenorhabditis elegans. Foods. 2024; 13(4):514. https://doi.org/10.3390/foods13040514
Chicago/Turabian StyleYu, Deyang, Meng Guo, Mingqian Tan, and Wentao Su. 2024. "Lipid-Lowering and Antioxidant Effects of Self-Assembled Astaxanthin–Anthocyanin Nanoparticles on High-Fat Caenorhabditis elegans" Foods 13, no. 4: 514. https://doi.org/10.3390/foods13040514