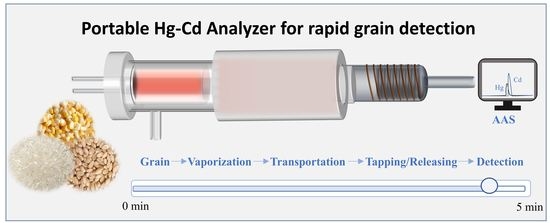
Rapid and Portable Detection of Hg and Cd in Grain Samples Based on Novel Catalytic Pyrolysis Composite Trap Coupled with Miniature Atomic Absorption Spectrometry
Abstract
:1. Introduction
2. Experimental
2.1. Instrumentation
2.2. Reagents, Materials, and Samples
2.3. Analytical Procedures
3. Results and Discussion
3.1. Design and Effect of Miniaturized AAS
3.2. Effect of ETV and Catalytic Unit for Grain Samples
3.3. Hg and Cd Trapping and Releasing by Pt/Ni Trap
3.4. Working Gas
3.5. Interference Study
3.6. Analytical Performance of the Method and Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, D.Y.; Gao, H.C.; Guo, N.; Jiang, M.M.; Ma, H.X.; Li, Y.C.; Li, Y.S.; Yang, Y.; Fang, G.X.; Zhu, K.T.; et al. Heavy Metals in Grains from Jilin Province, China, and Human Health Risk. J. Food Protect. 2020, 83, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Li, X.; Yu, L.; Wang, T.Q.; Wang, J.N.; Liu, T.T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IRAC Monographs on the Identification of Carcinogenic Hazards to Humans, Agents Classified by the IARC Monographs. Volumes 58. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 9 April 2023).
- Hu, Y.N.; Cheng, H.F.; Tao, S. The Challenges and Solutions for Cadmium-contaminated Rice in China: A Critical Review. Environ. Int. 2016, 92–93, 515–532. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Substance Priority List: The ATSDR 2022 Substance Priority List. Available online: http://www.atsdr.cdc.gov/SPL/index.html (accessed on 9 April 2023).
- Wang, J.X.; Feng, X.B.; Anderson, C.W.N.; Xing, Y.; Shang, L.H. Remediation of mercury contaminated sites- A review. J. Hazard. Mater. 2016, 221–222, 1–18. [Google Scholar] [CrossRef]
- Division of Food Safety Standards and Monitoring and Evaluation. Available online: http://www.nhc.gov.cn/sps/s7891/201301/16482cd1dec04196a4037d86104dcca9.shtml (accessed on 9 April 2023).
- An Official Website of the European Union, Document 32014R0488. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32014R0488 (accessed on 9 April 2023).
- Sharma, N.; Singh, V.K.; Lee, Y.; Kumar, S.; Rai, P.K.; Pathak, A.K.; Singh, V.K. Analysis of Mineral Elements in Medicinal Plant Samples Using LIBS and ICP-OES. Atom. Spectrosc. 2020, 41, 234–241. [Google Scholar] [CrossRef]
- Donato, F.D.; Biancolillo, A.; Foschi, M.; Cecco, V.D.; Martino, L.D.; D’Archivio, A.A. Authentication of typical Italian bell pepper spices by ICP-OES multi-elemental analysis combined with SIMCA class modelling. J. Food Compos. Anal. 2023, 115, 104948. [Google Scholar] [CrossRef]
- Pasias, I.N.; Rousis, N.I.; Psoma, A.K.; Thomaidis, N.S. Simultaneous or Sequential Multi-element Graphite Furnace Atomic Absorption Spectrometry Techniques: Advances Within the Last 20 Years. Atom. Spectrosc. 2021, 42, 310–327. [Google Scholar] [CrossRef]
- Alp, O.; Tosun, G. A rapid on-line non-chromatographic hydride generation atomic fluorescence spectrometry technique for speciation of inorganic arsenic in drinking water. Food Chem. 2019, 90, 10–15. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, R.; Zhang, W.; Yi, R.X.; Tang, S.S.; Guo, L.B.; Hao, Z.Q.; Li, X.Y.; Lu, Y.F.; Zeng, X.Y. High-sensitivity determination of cadmium and lead in rice using laser-induced breakdown spectroscopy. Food Chem. 2019, 272, 323–328. [Google Scholar] [CrossRef]
- Ravansari, R.; Wilson, S.C.; Tighe, M. Portable X-ray fluorescence for environmental assessment of soils: Not just a point and shoot method. Environ. Int. 2020, 134, 105250. [Google Scholar] [CrossRef]
- Li, K.; Wu, X.; Chen, Z.M.; Luo, J.; Hou, X.D.; Jiang, X.M. A simple dilution method for the direct determination of trace nickel in crude oil with a miniaturized electrothermal atomic absorption spectrometer. J. Anal. Atom. Spectrom. 2020, 35, 2656–2662. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Z.J.; Wang, Y.Y.; Zhang, X.N.; Yu, K.; Zhang, H.; Zhang, J.; Gao, J.; Liu, X.Y.; Zhang, H.N.; et al. Rapid determination of cadmium in rice by portable dielectric barrier discharge-atomic emission spectrometer. Food Chem. 2020, 310, 125824. [Google Scholar] [CrossRef]
- Belarra, M.A.; Resano, M.; Vanhaecke, F.; Moens, L. Direct solid sampling with electrothermal vaporization/atomization: What for and how? TrAC Trend Anal. Chem. 2002, 21, 828–839. [Google Scholar] [CrossRef]
- Zhong, G.P.; Luo, H.; Zhou, Z.D.; Hou, X.D. Molybdenum, platinum, and tantalum metal atomizers or vaporizers in analytical atomic spectrometry. Appl. Spectrosc. Rev. 2004, 39, 475–507. [Google Scholar] [CrossRef]
- Virgilio, A.; Healy, C.K.; Nóbrega, J.A.; Jones, B.T.; Donati, G.L. Evaluation of atomizer conditioning and pyrolysis and atomization temperature control to improve procedures based on tungsten coil atomic emission spectrometry. Microchem. J. 2013, 110, 758–763. [Google Scholar] [CrossRef]
- Hanna, S.N.; Jones, B.T. A review of tungsten coil electrothermal vaporization as a sample introduction technique in atomic spectrometry. Appl. Spectrosc. Rev. 2011, 46, 624–635. [Google Scholar] [CrossRef]
- Lv, Z.H.; Liu, J.X.; Mao, X.F.; Na, X.; Qian, Y.Z. Portable and miniature mercury analyzer using direct sampling inbuilt-metal ceramic electrothermal vaporization. Anal. Chim. Acta. 2022, 1231, 340444. [Google Scholar] [CrossRef]
- Liu, T.P.; Liu, J.X.; Huang, X.D.; Mao, X.F.; Qian, Y.Z. Novel Platinum-Nickel Composite Trap for Simultaneous and Direct Determination of Mercury and Cadmium in Soil and Its Mechanism Study. Anal. Chem. 2023, 95, 594–601. [Google Scholar] [CrossRef]
- Wang, B.; Feng, L.; Mao, X.F.; Liu, J.X.; Yu, C.C.; Ding, L.; Li, S.Q.; Zheng, C.M.; Qian, Y.Z. Direct determination of trace mercury and cadmium in food by sequential electrothermal vaporization atomic fluorescence spectrometry using tungsten and gold coil traps. J. Anal. Atom. Spectrom. 2018, 33, 1209–1216. [Google Scholar] [CrossRef]
- Mao, X.F.; Qi, Y.H.; Huang, J.W.; Liu, J.X.; Chen, G.Y.; Na, X.; Wang, M.; Qian, Y.Z. Ambient-Temperature Trap/Release of Arsenic by Dielectric Barrier Discharge and Its Application to Ultratrace Arsenic Determination in Surface Water Followed by Atomic Fluorescence Spectrometry. Anal. Chem. 2016, 88, 4147–4152. [Google Scholar] [CrossRef]
- Qi, Y.H.; Mao, X.F.; Liu, J.X.; Na, X.; Chen, G.Y.; Liu, M.T.; Zheng, C.M.; Qian, Y.Z. In Situ Dielectric Barrier Discharge Trap for Ultrasensitive Arsenic Determination by Atomic Fluorescence Spectrometry. Anal. Chem. 2018, 90, 6332–6338. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, J.X. Solid sampling graphite fibre felt electrothermal atomic fluorescence spectrometry with tungsten coil atomic trap for the determination of cadmium in food samples. J. Anal. Atom. Spectrom. 2010, 25, 1072–1078. [Google Scholar] [CrossRef]
- Mao, X.F.; Zhang, Y.; Liu, J.X.; Wang, M.; Qian, Y.Z.; Zhang, Z.W.; Qi, Y.H.; Gao, C.L. Simultaneous trapping of Zn and Cd by a tungsten coil and its application to grain analysis using electrothermal inductively coupled plasma mass spectrometry. RSC Adv. 2016, 6, 48699. [Google Scholar] [CrossRef]
- Liu, T.P.; Liu, J.X.; Mao, X.F.; Na, X.; Shao, Y.B.; Qian, Y.Z. Novel nickel trap coupled with electrothermal vaporization for the direct solid-sampling analysis of cadmium in soil. J. Anal. Atom. Spectrom. 2022, 37, 1611–1617. [Google Scholar] [CrossRef]
- Resano, M.; Vanhaecke, F.; de Loos-Vollebregt, M.T.C. Electrothermal vaporization for sample introduction in atomic absorption, atomic emission and plasma mass spectrometry—A critical review with focus on solid sampling and slurry analysis. J. Anal. Atom. Spectrom. 2008, 23, 1450–1475. [Google Scholar] [CrossRef]
- Lin, T.; Hu, J.; Deng, Y.J.; Zeng, W.; Jiang, X.M.; Hou, X.D. Development of a miniaturized hydride generation-dielectric barrier discharge atomic absorption spectrometer. Anal. Chim. Acta 2022, 1229, 340324. [Google Scholar] [CrossRef]
- Danet, A.F.; Bratu, M.C.; Radulescu, M.C.; Bratu, A. Portable minianalyzer based on cold vapor atomic absorption spectrometry at 184.9 nm for atmospheric mercury determination. Sens. Actuators B Chem. 2009, 137, 12–26. [Google Scholar] [CrossRef]
- Kelly, J.G.; Han, F.X.; Su, Y.; Xia, Y.; Philips, V.; Shi, Z.; Monts, D.L.; Pichardo, S.T.; Xia, K. Rapid determination of mercury in contaminated soil and plant samples using portable mercury direct analyzer without sample preparation, a comparative study. Water Air Soil Poll. 2012, 223, 2361–2371. [Google Scholar] [CrossRef]
- da Silva, M.J.; Paim, A.P.; Pimentel, M.F.; Cervera, M.L.; de la Guardia, M. Determination of total mercury in nuts at ultratrace level. Anal. Chim. Acta 2014, 838, 13–19. [Google Scholar] [CrossRef]
- Cizdziel, J.V.; Tolbert, C.; Brown, G. Direct analysis of environmental and biological samples for total mercury with comparison of sequential atomic absorption and fluorescence measurements from a single combustion event. Spectrochim. Acta B 2010, 65, 176–180. [Google Scholar] [CrossRef]
- Jora, M.Z.; Nóbrega, J.A.; Rohwedder, J.J.R.; Pasquini, C. Double tungsten coil atomic absorption spectrometer based on an acousto-optic tunable filter. Spectrochim. Acta B 2015, 103–104, 1–8. [Google Scholar] [CrossRef]
- Shakya, A.K.; Singh, S. State of the art in fiber optics sensors for heavy metals detection. Opt. Laser Technol. 2022, 153, 108246. [Google Scholar] [CrossRef]
- Ro, K.W.; Lim, K.; Shim, B.C.; Hahn, J.H. Integrated Light Collimating System for Extended Optical-Path-Length Absorbance Detection in Microchip-Based Capillary Electrophoresis. Anal. Chem. 2005, 77, 5160–5166. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.Y.; Pan, J.; Zhang, Z.; Zhang, L.; Tong, L.M. Coiled Optical Nanofiber for Optofluidic Absorbance Detection. ACS Sens. 2019, 4, 2267–2271. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.Z.; Li, X.; Feng, L.; Mao, X.F. Novel solid sampling electrothermal vaporization atomic absorption spectrometry for fast detection of cadmium in grain samples. J. Anal. Atom. Spectrom. 2021, 36, 285–293. [Google Scholar] [CrossRef]
- Shah, A.Q.; Kazi, T.G.; Baig, J.A.; Afridi, H.I.; Kandhro, G.A.; Arain, M.B.; Kolachi, N.F.; Wadhwa, S.K. Total mercury determination in different tissues of broiler chicken by using cloud point extraction and cold vapor atomic absorption spectrometry. Food Chem. Toxicol. 2010, 48, 65–69. [Google Scholar] [CrossRef]
- Aramendía, M.; Guarda, A.; Leite, D.; Resano, M. Direct mercury determination in blood and urine by means of high-resolution continuum source graphite furnace atomic absorption spectrometry using gold nanoparticles as a chemical modifier. J. Anal. Atom. Spectrom. 2017, 32, 2352–2359. [Google Scholar] [CrossRef]
- Liu, T.P.; Liu, M.T.; Liu, J.X.; Mao, X.F.; Zhang, S.S.; Shao, Y.B.; Na, X.; Chen, G.Y.; Qian, Y.Z. On-line microplasma decomposition of gaseous phase interference for solid sampling mercury analysis in aquatic food samples. Anal. Chim. Acta 2020, 1121, 42–49. [Google Scholar] [CrossRef]
- Guérin, T.; Chekri, R.; Chafey, C.; Testu, C.; Hulin, M.; Noël, L. Mercury in foods from the first French total diet study on infants and toddlers. Food Chem. 2018, 239, 920–925. [Google Scholar] [CrossRef]
- Djermanovic, M.; Baralic, I.; Pejic, S. Total Mercury Levels in Commercial Fish in Market of the Republic of Srpska, Bosnia and Herzegovina. Biol. Trace Elem. Res. 2020, 194, 545–551. [Google Scholar] [CrossRef]
- Şahan, S.; Şahin, U. An automated on-line minicolumn preconcentration cold vapour atomic absorption spectrometer: Application to determination of cadmium in water samples. Talanta 2012, 88, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.E.; Toro, J.A.; Briceño, M.; Rivas, R.E. Use of slow atomization ramp in high resolution continuum source graphite furnace atomic absorption spectrometry for the simultaneous determination of Cd and Ni in slurry powdered chocolate samples. Talanta 2022, 247, 123547. [Google Scholar] [CrossRef] [PubMed]






| Program | Power/W | Time/s | Gas | Signal Acquisition |
|---|---|---|---|---|
| Dehydration/Hg Vaporization | 30 | 40 | Air | No |
| Further ashing | 50 | 100 | Air | No |
| Gas switching | 0 | 15 | Ar/H2 | No |
| Cd vaporization | 100 | 40 | Ar/H2 | No |
| Release | 140 | 25 | Ar/H2 | Yes |
| Clean | 150 | 40 | Ar/H2 | No |
| Samples | Certified or Standard Method a (µg/kg) | This Method (µg/kg) | Added (μg/kg) | Measured Values (µg/kg) | Average Recoveries (%) b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hg | Cd | Hg | Cd | Hg | Cd | Hg | Cd | Hg | Cd | |
| Rice (GBW(E)100351) | 3.6 ± 0.9 | 420 ± 20 | 3.8 ± 0.8 | 419 ± 17 | / | / | / | / | / | / |
| Rice (GBW(E)100360) | 3 ± 1 | 220 ± 20 | 3.6 ± 0.7 | 226 ± 15 | / | / | / | / | / | / |
| Wheat (GSB-24) | 2.4 ± 0.3 | 18 ± 2 | NQ | 18 ± 1 | 10 | 10 | 13 ± 1 | 28 ± 2 | - | 98 |
| Corn (GSB-3) | 1.7 ± 0.2 | 4.1 ± 1.6 | NQ | 4.1 ± 0.6 | 10 | 10 | 12 ± 1 | 15 ± 1 | - | 106 |
| Rice (R-01) | 2.4 ± 0.4 | 56 ± 3 | NQ | 54 ± 3 | 10 | 100 | 12 ± 1 | 160 ± 7 | - | 106 |
| Rice (R-02) | 22 ± 1 | 433 ± 25 | 22 ± 2 | 444 ± 11 | 50 | 500 | 71 ± 4 | 968 ± 60 | 97 | 105 |
| Rice (R-03) | 44 ± 1 | 870 ± 26 | 44 ± 2 | 880 ± 28 | 50 | 500 | 94 ± 6 | 1356 ± 65 | 101 | 96 |
| Wheat (W-01) | 7.4 ± 0.5 | 106 ± 3 | 7.1 ± 0.8 | 104 ± 3 | 10 | 100 | 18 ± 1 | 215 ± 6 | 102 | 111 |
| Corn (C-01) | 12 ± 1 | 226 ± 4 | 12 ± 1 | 224 ± 5 | 50 | 500 | 64 ± 4 | 748 ± 20 | 103 | 105 |
| Method | Elements | Sampling Mode | Method LOD (μg/kg) | RSD (%) | Sample | Sample Size (mg) | Instrumental Weight and Power | Instrument Model and Details | Analysis Time (min) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| CPE-CV-AAS | Hg | Liquid | 0.117 | <5 | Broiler chicken | 1.5 mL | 200 kg; 5 kW | Aanalyst700; Desktop | / | [40] |
| HR CS GFAAS | Hg | Liquid | 2.3 | 15 | Blood, urine | / | 200 kg; 2.1 kW | ContrAA700; Desktop | <2 | [41] |
| ETV-DBD-GT-AFS | Hg | Solid | 0.5 | 10 | Aquatic food | 2–12 | 60 kg; 1.3 kW | Modified from DCMA200; Desktop | 5 | [42] |
| Direct mercury analyzer | Hg | Solid | LOQ: 0.6 | <5.8 | Food | 300 | 65 kg; 1.8 kW | AMA 254; Desktop | <6 | [43] |
| Direct mercury analyzer | Hg | Solid | 1 ng | / | Fish | 50 | 56 kg; 2 kw | DMA-80; Desktop | <5 | [44] |
| CV-AAS | Cd | Liquid | 2.1 ng/L | 2.5 | Water | 2.8 mL | 200 kg; 5 kW | Aanalyst700 Desktop | / | [45] |
| GF AAS | Cd | Slurry | 0.09 | 20 | Chocolate | 100 | 200 kg; 2 kW | ContrAA800 Desktop | <2 | [46] |
| ETV-AAS | Cd | Solid | 0.015 | 10 | Grain | 200 | 40 kg; 2 kW | Homemade Desktop | 3 | [39] |
| ETV-TC-APGD-AES | Cd | Liquid | 11.9 | 5.8 | Rice | 5 μL | 4.5 kg; 37 W | Homemade; Portable | 3 | [16] |
| Hg–Cd analyzer (Tungsten and gold coil traps) AFS | Hg, Cd | Solid | Hg: 0.07; Cd: 0.05 | 15 | Food | 10 | 60 kg; 1.5 kW | DCMA200; Desktop | 3 | [23] |
| MCH-CPF-Pt/Ni-AAS | Hg, Cd | Solid | Hg: 1.1; Cd: 0.3 | 20 | Grain | 15 | 14 kg; 270 W | Homemade; Portable | 5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Liu, J.; Mao, X.; Jiang, X.; Zhao, Y.; Qian, Y. Rapid and Portable Detection of Hg and Cd in Grain Samples Based on Novel Catalytic Pyrolysis Composite Trap Coupled with Miniature Atomic Absorption Spectrometry. Foods 2023, 12, 1778. https://doi.org/10.3390/foods12091778
Liu T, Liu J, Mao X, Jiang X, Zhao Y, Qian Y. Rapid and Portable Detection of Hg and Cd in Grain Samples Based on Novel Catalytic Pyrolysis Composite Trap Coupled with Miniature Atomic Absorption Spectrometry. Foods. 2023; 12(9):1778. https://doi.org/10.3390/foods12091778
Chicago/Turabian StyleLiu, Tengpeng, Jixin Liu, Xuefei Mao, Xiaoming Jiang, Yabo Zhao, and Yongzhong Qian. 2023. "Rapid and Portable Detection of Hg and Cd in Grain Samples Based on Novel Catalytic Pyrolysis Composite Trap Coupled with Miniature Atomic Absorption Spectrometry" Foods 12, no. 9: 1778. https://doi.org/10.3390/foods12091778