Microbial Diversity and Correlation between Breast Milk and the Infant Gut
Abstract
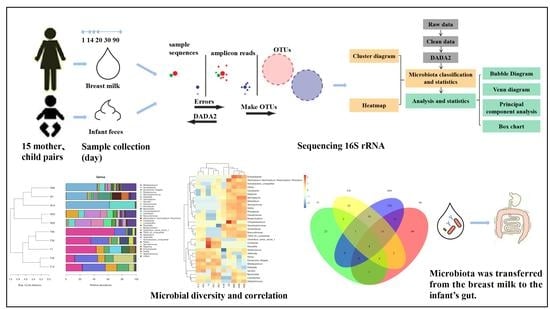
:1. Introduction
2. Methods
2.1. Participants
2.2. Sample Collection
2.3. DNA Extraction and Illumina Sequencing
2.4. Bioinformatics and Statistical Analysis
3. Results
3.1. Sequencing Summary
3.2. Diversity of Breast Milk and Infant Intestinal Microbiota
3.2.1. Cluster Analysis of Human Milk and Infant Feces at the Genus and Species Levels
3.2.2. Heatmap Analysis of Human Milk and Infant Feces at the Genus and Species Levels
3.2.3. Bubble Chart Analysis of Human Milk and Infant Feces for Detecting Microbiota
3.3. Relationship between Breast Milk and Infant Intestinal Microbiota
3.3.1. Venn Diagram of the Microbiota in Human Milk and Infant Feces
3.3.2. Principal Component Analysis of Microbiota in Human Milk and Infant Fecal Samples
3.3.3. Box Diagram of Microbiota in Human Milk and Infant Feces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selma-Royo, M.; Calvo-Lerma, J.; Cortes-Macias, E.; Carmen-Collado, M. Human Milk Microbiome: From actual knowledge to future perspective. Semin Perinatol. 2021, 45, 151450. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.G.; Christensen, S.H.; Lind, M.V.; Michaelsen, K.F. Human milk composition and infant growth. Curr. Opin. Clin. Nutr. 2018, 21, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Fontana, L.; Gil, A. Human milk oligosaccharides and immune system development. Nutrients 2018, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Giannì, M.L. Human milk: Composition and health benefits. La Pediatr. E Chir. Med. Surg. Pediatr. 2017, 39, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Mazzoni, C.; Hogstrom, L.; Bryant, A.; Bergerat, A.; Cher, A.; Pochan, S.; Herman, P.; Carrigan, M.; Sharp, K.; et al. Delivery mode affects stability of early infant gut microbiota. Cell Rep. Med. 2020, 1, 100156. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Stanton, C.; Paul-Ross, R.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium and lactobacillus composition at species level and gut microbiota diversity in infants before 6 weeks. Int. J. Mol. Sci. 2019, 20, 3306. [Google Scholar] [CrossRef]
- Lyu, L.; Zhou, X.; Zhang, M.; Liu, L.; Niu, H.; Zhang, J.; Chen, S.; Gong, P.; Jiang, S.; Pan, J.; et al. Delivery Mode Affects Intestinal Microbial Composition and the Development of Intestinal Epithelial Cells. Front. Microbiol. 2021, 12, 626144. [Google Scholar] [CrossRef]
- Granger, C.L.; Embleton, N.D.; Palmer, J.M.; Lamb, C.A.; Berrington, J.E.; Stewart, C.J. Maternal breastmilk, infant gut microbiome and the impact on preterm infant health. Acta Paediatr. 2021, 110, 450–457. [Google Scholar] [CrossRef]
- Biagi, E.; Quercia, S.; Aceti, A.; Beghetti, I.; Rampelli, S.; Turroni, S.; Faldella, G.; Candela, M.; Brigidi, P.; Corvaglia, L. The bacterial ecosystem of mother’s milk and infant’s mouth and gut. Front. Microbiol. 2017, 8, 1214. [Google Scholar] [CrossRef]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The gut microbiota in the first decade of life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef] [PubMed]
- Ficara, M.; Pietrella, E.; Spada, C.; Muttini, E.D.C.; Lucaccioni, L.; Iughetti, L.; Berardi, A. Changes of intestinal microbiota in early life. J. Matern. Fetal Neonatal Med. 2020, 33, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; de Vos, W.M. Early life colonization of the human gut: Microbes matter everywhere. Curr. Opin. Microbiol. 2018, 44, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Khodayar-Pardo, P.; Mira-Pascual, L.; Collado, M.C.; Martínez-Costa, C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol. 2014, 34, 599–605. [Google Scholar] [CrossRef]
- Kumar, H.; Du Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.L.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C.; et al. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front. Microbiol. 2016, 7, 1619. [Google Scholar] [CrossRef]
- Lackey, K.A.; Williams, J.E.; Meehan, C.L.; Zachek, J.A.; Benda, E.D.; Price, W.J.; Foster, J.A.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; et al. What’s normal? Microbiomes in human milk and infant feces are related to each other but vary geographically: The INSPIRE study. Front. Nutr. 2019, 6, 45. [Google Scholar] [CrossRef]
- Lee, M.K.; Binns, C. Breastfeeding and the risk of infant illness in Asia: A review. Int. J. Environ. Res. Public Health 2020, 17, 186. [Google Scholar] [CrossRef]
- Nuzzi, G.; Di Cicco, M.E.; Peroni, D.G. Breastfeeding and allergic diseases: What’s new? Children 2021, 8, 330. [Google Scholar] [CrossRef]
- Liu, J. Exploration of the Effect of Breastfeeding on the Metabolism of Intestinal Flora in Infants; Soochow University: Suzhou, China, 2019; pp. 22–31. [Google Scholar] [CrossRef]
- Murphy, K.; Curley, D.; O’Callaghan, T.F.; O’Shea, C.; Dempsey, E.M.; O’Toole, P.W.; Paul Ross, R.; Ryan, C.A.; Stanton, C. The composition of human milk and infant faecal microbiota over the first three months of life: A pilot study. Sci. Rep. 2017, 7, 40597. [Google Scholar] [CrossRef]
- Yassour, M.; Jason, E.; Hogstrom, L.J.; Arthur, T.D.; Tripathi, S.; Siljander, H.; Selvenius, J.; Oikarinen, S.; Hyöty, H.; Virtanen, S.M.; et al. Strain-level analysis of mother-to-child bacterial transmission during the first few months of life. Cell Host Microbe 2018, 24, 146–154.e4. [Google Scholar] [CrossRef]
- Yang, R.; Gao, R.; Cui, S.; Zhong, H.; Zhang, X.; Chen, Y.; Wang, J.; Qin, H. Dynamic signatures of gut microbiota and influences of delivery and feeding modes during the first 6 months of life. Physiol. Genom. 2019, 51, 368–378. [Google Scholar] [CrossRef]
- Nagpal, R.; Tsuji, H.; Takahashi, T.; Kawashima, K.; Nagata, S.; Nomoto, K.; Yamashiro, Y. Sensitive quantitative analysis of the meconium bacterial microbiota in healthy term infants born vaginally or by cesarean section. Front. Microbiol. 2016, 7, 1997. [Google Scholar] [CrossRef]
- Hermansson, H.; Kumar, H.; Collado, M.C.; Salminen, S.; Isolauri, E.; Rautava, S. Breast milk microbiota is shaped by mode of delivery and intrapartum antibiotic exposure. Front. Nutr. 2019, 6, 4. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Peroni, D.G.; Grossi, E.; Facchin, V.; Comberiati, P.; Drago, L. Impact of delivery mode on the colostrum microbiota composition. BMC Microbiol. 2017, 17, 205. [Google Scholar] [CrossRef]
- Leyva, L.L.; Brereton, N.J.B.; Koski, K.G. Emerging frontiers in human milk microbiome research and suggested primers for 16S rRNA gene analysis. Comput. Struct. Biotechnol. 2021, 19, 121–133. [Google Scholar] [CrossRef]
- Xi, X. Study on the Correlation between the Intestinal Flora of Newborn Infants and the Flora of Various Maternal Sites; Inner Mongolia Agricultural University: Huhehaote, China, 2017; pp. 13–16. [Google Scholar]
- Layuk, N.; Sinrang, A.W.; Asad, S. Early initiation of breastfeeding and gut microbiota of neonates: A literature review. Med. Práctica 2021, 4, 100222. [Google Scholar] [CrossRef]
- Boudry, G.; Charton, E.; Huerou-Luron, L.; Ferret-Bernard, S.; Gall, S.L.; Even, S.; Blat, S. The relationship between breast milk components and the infant gut microbiota. Front. Nutr. 2021, 8, 87. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Mantziari, A.; Rautava, S. Factors influencing the microbial composition of human milk. Semin Perinatol. 2021, 45, 151507. [Google Scholar] [CrossRef]
- Frank, N.M.; Lynch, K.F.; Uusitalo, U.; Yang, J.; Lönnrot, M.; Virtanen, S.M.; Hyöty, H.; Norris, J.M. The relationship between breastfeeding and reported respiratory and gastrointestinal infection rates in young children. BMC Pediatr. 2019, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Fente, C.; Regal, P.; Lamas, A.; Lorenzo, M.P. Human Milk Oligosaccharides (HMOs) and Infant Microbiota: A Scoping Review. Foods 2021, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.E.; Meier, A.K.; Cernioglo, K.; Mitchell, R.D.; Casaburi, G.; Frese, S.A.; Henrick, B.M.; Underwood, M.A.; Smilowitz, J.T. Early probiotic supplementation with B. infantis in breastfed infants leads to persistent colonization at 1 year. Pediatr. Res. 2022, 91, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef]







| Mother’s Serial Number | Sample Days | Mother’s Age | Delivery Times | Delivery Mode | Use of Antibiotics | Infant Feeding Mode |
|---|---|---|---|---|---|---|
| 1 | 1 d | 30 | Firstborn | Cesarean section | Unused | Breast-fed and formula-fed |
| 2 | 1 d | 32 | Firstborn | Cesarean section | Unused | Breast-fed and formula-fed |
| 3 | 1 d | 26 | Firstborn | Vaginal delivery | Unused | Breast-fed and formula-fed |
| 4 | 14 d | 34 | Firstborn | Cesarean section | Unused | Breast-fed |
| 5 | 14 d | 28 | Firstborn | Vaginal delivery | Unused | Breast-fed |
| 6 | 14 d | 27 | Firstborn | Cesarean section | Unused | Breast-fed |
| 7 | 20 d | 26 | Firstborn | Cesarean section | Unused | Breast-fed |
| 8 | 20 d | 28 | Firstborn | Cesarean section | Unused | Breast-fed |
| 9 | 20 d | 29 | Firstborn | Cesarean section | Unused | Breast-fed |
| 10 | 30 d | 30 | Firstborn | Vaginal delivery | Unused | Breast-fed |
| 11 | 30 d | 31 | Firstborn | Cesarean section | Unused | Breast-fed |
| 12 | 30 d | 30 | Firstborn | Cesarean section | Unused | Breast-fed |
| 13 | 90 d | 33 | Firstborn | Cesarean section | Unused | Breast-fed |
| 14 | 90 d | 29 | Firstborn | Cesarean section | Unused | Breast-fed |
| 15 | 90 d | 25 | Firstborn | Vaginal delivery | Unused | Breast-fed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Xia, X.; Sun, L.; Wang, H.; Li, Q.; Yang, Z.; Ren, J. Microbial Diversity and Correlation between Breast Milk and the Infant Gut. Foods 2023, 12, 1740. https://doi.org/10.3390/foods12091740
Wang K, Xia X, Sun L, Wang H, Li Q, Yang Z, Ren J. Microbial Diversity and Correlation between Breast Milk and the Infant Gut. Foods. 2023; 12(9):1740. https://doi.org/10.3390/foods12091740
Chicago/Turabian StyleWang, Kaili, Xiufang Xia, Lina Sun, Hui Wang, Qiu Li, Zhuo Yang, and Jing Ren. 2023. "Microbial Diversity and Correlation between Breast Milk and the Infant Gut" Foods 12, no. 9: 1740. https://doi.org/10.3390/foods12091740