Effects of Four Strains of Actinomycetes on the Content of Terpenoids in Baijiu
Abstract
:1. Introduction
2. Materials and Methods
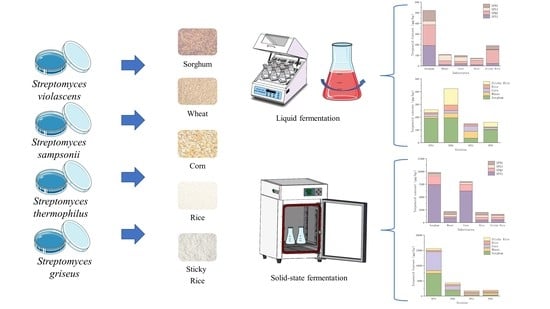
2.1. Microorganisms and Media
2.2. Fermentation Conditions
2.2.1. Solid-State Fermentation Conditions
2.2.2. Liquid-State Fermentation Conditions
2.3. Semi-Quantitative Analysis of Terpenoids Using Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry (HS-SPME-GC–MS)
2.3.1. Solid-State Fermentation Sample Processing
2.3.2. Liquid-State Fermentation Sample Processing
2.3.3. HS-SPME Conditions
2.3.4. GC-MS Conditions
2.4. Data Analysis
3. Results
3.1. Analysis of the Liquid Fermentation Terpenoid Metabolites
3.1.1. Comparative Analysis of the Products of Different Substrates
3.1.2. Comparative Analysis of the Products of Different Strains
3.2. Analysis of the Solid-State Fermentation Terpenoid Metabolites
3.2.1. Comparative Analysis of the Products of Different Substrates
3.2.2. Comparative Analysis of the Products of Different Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.W.; Han, B.Z. Baijiu, Chinese liquor: History, classification and manufacture. J. Ethn. Foods 2016, 3, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Fan, Z.; Du, A.; Li, Y.; Zhang, R.; Shi, Q.; Shi, L.; Chu, X. Recent advances in Baijiu analysis by chromatography based technology-A review. Food Chem. 2020, 324, 126899. [Google Scholar] [CrossRef]
- Li, X.R.; Ma, E.B.; Yan, L.Z.; Meng, H.; Du, X.W.; Zhang, S.W.; Quan, Z.X. Bacterial and fungal diversity in the traditional Chinese liquor fermentation process. Int. J. Food Microbiol. 2011, 146, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Tian, W.; Zhao, D. Research progress of trace components in sesame-aroma type of baijiu. Food Res. Int. 2020, 137, 109695. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhi, H.; Fang, Z.; Zhang, P. Genetic engineering of yeast, filamentous fungi and bacteria for terpene production and applications in food industry. Food Res. Int. 2021, 147, 110487. [Google Scholar] [CrossRef] [PubMed]
- Dziadas, M.; Jeleń, H.H. Analysis of terpenes in white wines using SPE–SPME–GC/MS approach. Anal. Chim. Acta 2010, 677, 43–49. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y. Review of important functional compounds terpenes in Baijiu (Chinese Liquor). Liquor Mak. 2013, 2013, 11–16. [Google Scholar]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Howell, K.; Fang, Z.; Zhang, P. Sesquiterpenes in grapes and wines: Occurrence, biosynthesis, functionality, and influence of winemaking processes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 247–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, Z.; Chen, J.; Zheng, Y.; Limsila, B.; Lu, M.; Gao, T.; Yang, Q.; Fu, C.; Liao, W. Terpenoids from Curcumae Rhizoma: Their anticancer effects and clinical uses on combination and versus drug therapies. Biomed. Pharmacother. 2021, 138, 111350. [Google Scholar] [CrossRef] [PubMed]
- Hortelano, S.; González-Cofrade, L.; Cuadrado, I.; Heras, B. Current status of terpenoids as inflammasome inhibitors. Biochem. Pharmacol. 2019, 172, 113739. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Rico, J.; Gil, J.V.; Orejas, M. De novo production of six key grape aroma monoterpenes by a geraniol synthase-engineered S. cerevisiae wine strain. Microb. Cell Factories 2015, 14, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Fan, W.; Xu, Y. Characterization of the key odorants in light aroma type Chinese liquor by gas chromatography–olfactometry, quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2014, 62, 5796–5804. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fan, W.; Wu, Q. Determination and mechanism of common and typical characteristics flavor of Chinese light aroma style liquors. Liquor. Mak. 2012, 39, 107–112. [Google Scholar]
- Wang, L.; Hu, G.; Lei, L.; Lin, L.; Wang, D.; Wu, J. Identification and aroma impact of volatile terpenes in Moutai liquor. Int. J. Food Prop. 2016, 19, 1335–1352. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, X.; Ji, K.; Guo, K.; Li, Y.; Wu, C.; Xu, G. Characterization of flavor compounds in Chinese liquor Moutai by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Anal. Chim. Acta 2007, 597, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, Y.; Mo, K.; Zhou, Z. Research on terpenes in Jiannanchun Liquor by GC×GC-TOFMS. Liquor. Mak. Sci. Technol. 2009, 9, 48–49. [Google Scholar]
- Hu, G.; Fan, W.; Xu, Y.; Jia, Q.; Ran, X. Research on terpenoids in dongjiu. Liquor. Mak. Sci. Technol. 2011, 7, 29–33. [Google Scholar]
- Fan, W.; Hu, G.; Xu, Y. Quantification of volatile terpenoids in Chinese medicinal liquor using headspace-solid phase microextraction coupled with gas chromatography-mass spectrometry. Food Sci. 2012, 33, 110–116. [Google Scholar]
- Madžgalj, V.; Petrović, A.; Čakar, U.; Maraš, V.; Sofrenić, I.; Tešević, V. The influence of different enzymatic preparations and skin contact time on aromatic profile of wines produced from autochthonous grape varieties Krstač and Žižak. J. Serb. Chem. Soc. 2023, 88, 11–23. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Q.; Li, J.; Xu, Y. Isolation and analysis of bound aroma compounds in different raw brewing materials. J. Food Sci. Biotechnol. 2015, 34, 456–462. [Google Scholar]
- Zhang, Z.; Yang, R.; Zhu, L.; Wu, X. Strategies for improving the yield of microbial terpenoids production. China Biotechnol. 2017, 37, 97–103. [Google Scholar]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically bound aroma precursors in fruits: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef]
- Du, H.; Fan, W.; Xu, Y. Characterization of geosmin as source of earthy odor in different aroma type Chinese liquors. J. Agric. Food Chem. 2011, 59, 8331–8337. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, L.; Lin, L.; Wang, H.; Wang, L. Isolation & identification of an actinomycetes strain and analysis of its metabolites. Liquor. Mak. Sci. Technol. 2014, 2014, 42–44. [Google Scholar]
- Liu, H.; Sun, B. Effect of fermentation processing on the flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Y.J.; Huang, J.; Hu, Y.; Zhang, Z.Y. Classification, production process and nutritional value of Chinese liquor. Agr. Eng. Technol. 2007, 1, 21–24. [Google Scholar]
- Luo, T.; Fan, W.; Xu, Y. Characterization of volatile and semi-volatile compounds in Chinese rice wines by headspace solid phase microextraction followed by gas chromatography-mass spectrometry. J. Inst. Brew. 2008, 114, 172–179. [Google Scholar] [CrossRef]
- Gamero, A.; Manzanares, P.; Querol, A.; Belloch, C. Monoterpene alcohols release and bioconversion by Saccharomyces species and hybrids. Int. J. Food Microbiol. 2011, 145, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Torrens, J.; Urpí, P.; Riu-Aumatell, M.; Vichi, S.; López-Tamames, E.; Buxaderas, S. Different commercial yeast strains affecting the volatile and sensory profile of cava base wine. Int. J. Food Microbiol. 2008, 124, 48–57. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, W.; Wang, W.; Xu, Y. Effect of yeast species on the terpenoids profile of Chinese light-style liquor. Food Chem. 2015, 168, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Barrios-González, J. Solid-state fermentation: Physiology of solid medium, its molecular basis and applications. Process. Biochem. 2012, 47, 175–185. [Google Scholar] [CrossRef]
- Tshering, G.; Plengsuriyakarn, T.; Na-Bangchang, K.; Pimtong, W. Embryotoxicity evaluation of atractylodin and β-eudesmol using the zebrafish model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 239, 108869. [Google Scholar] [CrossRef]
- Zini, C.A.; De Assis, T.F.; Ledford, E.B.; Dariva, C.; Fachel, J.; Christensen, E.; Pawliszyn, J. Correlations between pulp properties of eucalyptus clones and leaf volatiles using automated solid-phase microextraction. J. Agric. Food Chem. 2003, 51, 7848–7853. [Google Scholar] [CrossRef] [PubMed]
- de Alencar, D.C.; Pinheiro, M.L.B.; Pereira, J.L.D.S.; de Carvalho, J.E.; Campos, F.R.; Serain, A.F.; Tirico, R.B.; Tasco, A.J.H.; Costa, E.V.; Salvador, M.J. Chemical composition of the essential oil from the leaves of Anaxagorea brevipes (Annonaceae) and evaluation of its bioactivity. Nat. Prod. Res. 2016, 30, 1088–1092. [Google Scholar] [CrossRef]
- Ghazouani, N.; Sifaoui, I.; Bachrouch, O.; Abderrabba, M.; Pinero, J.E.; Lorenzo-Morales, J. Essential oil composition and anti Acanthamoeba studies of Teucrium ramosissimum. Exp. Parasitol. 2017, 183, 207–211. [Google Scholar] [CrossRef]
- Kishimoto, T.; Wanikawa, A.; Kagami, N.; Kawatsura, K. Analysis of hop-derived terpenoids in beer and evaluation of their behavior using the stir bar− sorptive extraction method with GC-MS. J. Agric. Food Chem. 2005, 53, 4701–4707. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Effects of an Atractylodes lancea rhizome extract and a volatile component β-eudesmol on gastrointestinal motility in mice. J. Ethnopharmacol. 2012, 141, 530–536. [Google Scholar] [CrossRef]
- Ohara, K.; Misaizu, A.; Kaneko, Y.; Fukuda, T.; Miyake, M.; Miura, Y.; Okamura, H.; Yajima, J.; Tsuda, A. β-eudesmol, an oxygenized sesquiterpene, reduces the increase in saliva 3-methoxy-4-hydroxyphenylglycol after the “Trier social stress test” in healthy humans: A randomized, double-blind, placebo-controlled cross-over study. Nutrients 2018, 11, 9. [Google Scholar] [CrossRef] [Green Version]






| Terpenoids | Control b | SPS1 | SPQ1 | SPG1 | SPH1 | |
|---|---|---|---|---|---|---|
| Sorghum | 1-Octen-3-ol | - c | - | - | 1.96 | - |
| (E)-2-Octenal | - | - | - | 2.43 | - | |
| Nerolidol | - | - | - | 2.2 | - | |
| Geranylacetone | - | - | - | 3.44 | - | |
| (+)-β-Cedrene | - | 35.34 | 15.69 | - | - | |
| β-Eudesmol | - | 146.54 | 166.47 | - | 62.05 | |
| Geranyl isovalerate | - | 0.53 | - | - | 0.43 | |
| Fitone | 3.40 | 9.24 | 10.56 | 3.62 | 31.72 | |
| (−)-Epiglobulol | - | - | 1.92 | - | - | |
| 2,4-Decadienal | - | - | - | 0.11 | - | |
| 2-Undecenal | - | - | - | 5.97 | - | |
| Zingiberene | - | - | - | 6.37 | - | |
| β-Sesquiphellandrene | - | - | - | 9.04 | - | |
| β-Elemene | - | - | - | - | 5.93 | |
| Wheat | Hinokitiol | - | 1.25 | - | 26.47 | - |
| Geraniol | - | 1.68 | - | - | - | |
| Linalool | - | 1.74 | 0.94 | - | 1.55 | |
| Nerolidol | - | 5.75 | - | - | - | |
| β-Eudesmol | - | - | 23.33 | - | - | |
| Geranyl isovalerate | - | - | - | 3.93 | - | |
| Fitone | 1.73 | 1.82 | 5.84 | 22.34 | 2.02 | |
| Cedrol | - | 0.34 | - | - | - | |
| Nerol | - | - | 5.67 | - | 1.27 | |
| (−)-Epicedrol | - | - | - | 1.01 | - | |
| Corn | (E)-2-Octenal | - | 3.62 | - | 4.42 | - |
| Nerolidol | 1.79 | 5.22 | 3.28 | 4.34 | 2.86 | |
| Geranylacetone | - | 6.48 | 4.55 | - | - | |
| Fitone | 0.14 | 1.48 | 0.51 | 0.9 | 1.1 | |
| Dihydro-β-ionone | 1.45 | 1.69 | 1.50 | 3.3 | 1.58 | |
| α-Acorenol | 1.40 | 1.95 | 1.58 | 1.41 | 1.56 | |
| 2-Undecenal | - | - | 7.16 | - | - | |
| Nerol | - | - | - | 13.04 | 5.23 | |
| β-Ionone | - | - | 3.16 | - | - | |
| Geranyl isovalerate | - | - | - | 0.1 | 1.84 | |
| Isocalamenediol | - | - | - | 0.14 | - | |
| (−)-Epicedrol | - | - | - | 0.33 | - | |
| Eremophilene | - | - | - | 2.06 | - | |
| α-Cadinol | - | - | - | 9.42 | - | |
| Rice | Nerol | - | 0.35 | - | - | 0.16 |
| (E)-2-Octenal | - | 7.42 | - | - | 2.87 | |
| Geranylacetone | 1.08 | 1.15 | 5.6 | 1.10 | 0.95 | |
| Fitone | 0.46 | 2.00 | 4.42 | 1.88 | 0.48 | |
| Linalool | - | - | 16.01 | 9.30 | - | |
| β-Eudesmol | - | - | 16.46 | - | - | |
| (−)-α-Terpineol | - | - | - | 3.13 | - | |
| Glutinousrice | (−)-Epicedrol | - | 0.20 | - | - | - |
| Terpinen-4-ol | - | 1.68 | - | - | - | |
| Linalool | 2.13 | 12.13 | 8.21 | 2.5 | 10.23 | |
| Citronellol | - | 2.55 | - | - | - | |
| 1-Octen-3-ol | - | 3.28 | 46.7 | - | 15.52 | |
| Geranylacetone | 0.31 | 0.39 | 3.93 | 0.49 | 0.52 | |
| Fitone | 0.54 | 2.20 | 2.62 | 0.8 | 2.06 | |
| 2,4-Decadienal | - | - | 10.8 | - | - | |
| Geraniol | - | - | - | 0.56 | 1.53 | |
| 2-Undecenal | - | - | 12.05 | - | - | |
| β-Eudesmol | - | - | 18.02 | - | - | |
| Farnesol | - | - | 2.79 | - | - | |
| (E)-2-Octenal | - | - | 23.9 | - | - | |
| (E)-2-Heptenal | - | - | - | - | 6.68 |
| Terpenoids | Sorghum | Wheat | Corn | Rice | Sticky Rice |
|---|---|---|---|---|---|
| (E)-2-Octenal | - b | - | 0.63 | - | - |
| Nerolidol | 4.62 | - | - | - | - |
| Fitone | 4.69 | 8.17 | 10.46 | 2.66 | 3.01 |
| Linalool | - | - | 0.77 | - | - |
| Geranylacetone | - | - | - | - | 1.25 |
| 7d a | 14d | 21d | 28d | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | 1 b | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| DL-Menthol | 180.23 | 549.42 | 305.75 | 129.94 | 60.36 | 401.04 | 120.21 | 115.36 | 17.48 | 21.47 | 1294.79 | 116.77 | 805.01 | 90.71 | 33.92 | 401.75 | 98.5 | 507.95 | 17.39 | 42.53 |
| Elemol | 44.64 | 72.69 | 2.82 | 65.11 | 14.79 | 572.1 | 117.99 | 1.51 | 9.03 | 20.01 | 176.92 | 16.13 | 7.73 | 19.82 | 13.46 | 76.59 | 29.35 | 12.86 | 18.9 | 0.66 |
| Linalool | 80.36 | 154.97 | 57.4 | 27.59 | 9.00 | 23.75 | 51.88 | 20.13 | 111.51 | 15.31 | 60.96 | 51.37 | 70.89 | 69.69 | 11.41 | 28.85 | 23.51 | 38.07 | 59.05 | 35.39 |
| (+)-β-Funebrene | 60.13 | - c | 44.89 | - | 0.55 | 11.5 | - | 38.98 | - | 0.09 | 887.77 | - | 304.84 | - | - | 447.77 | - | 208.95 | - | - |
| Cedrenol | 8.11 | 51.02 | 4.26 | - | - | 35.22 | 75.73 | 2.65 | - | - | 89.39 | 48.95 | 13.88 | - | - | 63.35 | 212.52 | 6.36 | - | - |
| Cedrene | 15.93 | 72.16 | 27.76 | 3.12 | 7.99 | 4.41 | 63.08 | 17.35 | 0.12 | 49.98 | 112.75 | 23.44 | 145.97 | 5.92 | 4.35 | 150.61 | 3.7 | 136.32 | 3.77 | 19.65 |
| 4(15),5,10(14)-Germacratrien-1-ol | 481.55 | 443.89 | 24.11 | 53.26 | 63.64 | 305.22 | 230.05 | 7.66 | 20.58 | 126.01 | 1612.96 | 679.72 | 5.29 | 30.94 | 162.34 | 544.49 | 75.49 | 43.69 | 62.11 | 85.69 |
| β-elemene | 28.74 | 133.40 | 2.23 | 34.22 | 3.91 | 33.38 | 148.46 | 0.96 | 53.02 | 12.21 | 356.69 | 98.94 | - | 21.78 | 4.26 | 71.85 | 7.49 | - | 11.89 | 13.41 |
| Nerolidol | 167.55 | 270.25 | 42.12 | 89.16 | 76.89 | 178.32 | 15.33 | 25.88 | 14.02 | 17.34 | 221.01 | 22.02 | 115.57 | 32.46 | 19.12 | 79.49 | 21.64 | 67.72 | 24.24 | 64.12 |
| Fitone | 149.82 | 278.94 | 98.00 | 104.38 | 34.12 | 72.75 | 183.53 | 40.28 | 39.85 | 26.76 | 562.49 | 271.19 | 394.36 | 100.74 | 46.12 | 349.5 | 105.15 | 355.93 | 73.46 | 79.66 |
| (+)-β-cedrene | 185.68 | 778.62 | 111.95 | 208.62 | 47.82 | 71.43 | 123.77 | 290.62 | 32.97 | 59.11 | 5354.58 | 133.37 | 3806.73 | 204.23 | 98.33 | 1807.29 | 33.58 | 3064.30 | 214.09 | 103.31 |
| β-eudesmol | 1239.08 | 10276.62 | 816.81 | 2907.81 | 687.46 | 1831.98 | 5149.35 | 1057.8 | 1244.12 | 941.11 | 9635.02 | 2679.17 | 5496.13 | 1781.23 | 2118.22 | 5397.31 | 1356.9 | 3093.88 | 1083.94 | 796.40 |
| Phytol | 0.55 | 1.58 | - | 2.92 | 0.42 | 2.73 | 0.84 | - | 1.03 | 0.18 | 2.73 | 1.61 | - | 1.94 | 1.14 | 1.15 | 0.92 | - | 0.12 | 5.28 |
| (E)-2-Octenal | 58.38 | 8.84 | 99.85 | 0.11 | 1.68 | 105.67 | 4.31 | 41.21 | 0.03 | 0.46 | 4.40 | 1.31 | 84.56 | - | - | 0.2 | 2.22 | 42.16 | - | - |
| Geranyl isovalerate | 6.87 | 19.94 | 3.06 | 4.73 | 1.25 | 2.91 | 4.26 | 0.67 | 1.00 | 3.85 | 4.52 | 2.33 | - | 2.83 | 0.74 | 4.40 | 0.79 | - | 0.17 | - |
| Nerol | 1.47 | 20.79 | - | 5.86 | - | 0.81 | 3.14 | - | 0.39 | - | 6.66 | - | - | 6.82 | - | 9.33 | - | - | 6.60 | - |
| α-Bulnesene | 54.83 | 389.99 | 115.67 | 132.62 | 2.5 | 17.01 | 379.89 | 31.48 | 106.21 | 13.61 | 40.80 | 74.62 | 217.72 | 105.15 | 2.14 | 70.05 | 14.45 | 121.63 | 32.78 | 36.06 |
| (−)-Globulol | 11.48 | - | - | - | - | 3.98 | - | - | - | - | 48.57 | - | - | - | - | 62.98 | - | - | - | - |
| α-Agarofuran | 1.11 | - | - | - | - | 0.33 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| δ-Cadinene | 39.89 | 112.58 | - | 12.31 | - | 97.69 | 69.42 | - | 63.57 | - | 119.74 | 8.09 | - | 43.52 | - | 20.19 | - | - | 34.16 | - |
| 2-Undecenal | 11.66 | - | 52.07 | - | - | 0.15 | - | 22.33 | - | - | - | - | 35.12 | - | - | - | - | 8.37 | - | - |
| β-Caryophyllene | 2.45 | - | - | - | - | 54.60 | - | - | - | - | 36.14 | - | - | - | - | 1.79 | - | - | - | - |
| (−)-Humulene epoxide II | 5.84 | - | - | - | - | 107.95 | - | - | - | - | 109.68 | - | - | - | - | 5.97 | - | - | - | - |
| Farnesal | 160.07 | 33.26 | 14.26 | - | - | 1214.38 | 143.51 | 0.35 | - | - | 224.94 | 31.55 | - | - | - | 4.00 | - | - | - | - |
| α-Farnesene | 17.32 | - | - | - | - | 162.85 | - | - | - | - | 143.13 | - | - | - | - | 6.79 | - | - | - | - |
| Cembrene | 56.63 | 38.40 | - | - | - | 240.18 | 88.07 | - | - | - | 37.6 | 48.94 | - | - | - | 28.11 | 11.00 | - | - | - |
| (E)-β-Famesene | 48.83 | - | - | - | - | 276.67 | - | - | - | - | 72.34 | - | - | - | - | 4.64 | - | - | - | - |
| α-Bisabolol | 5.89 | - | 352.76 | 15.26 | 20.84 | 131.47 | - | 22.58 | 1.23 | 29.80 | 507.05 | - | 27.94 | 16.73 | 19.02 | 80.04 | - | 166.06 | 40.77 | 53.66 |
| 1-Octen-3-ol | 111.44 | 14.35 | 532.74 | - | 6.76 | 1776.01 | 1118.52 | 124.24 | - | 1.20 | 1.68 | 2.04 | 227.3 | - | - | 0.15 | - | 42.41 | - | - |
| trans-Farnesol | 427.85 | - | - | 0.01 | - | 2872.72 | - | - | 0.89 | - | 923.86 | - | - | 11.00 | - | 51.08 | - | - | 17.91 | - |
| α-Gurjenene | 32.33 | - | - | - | - | 5.88 | - | - | - | - | 0.24 | - | - | - | - | - | - | - | - | - |
| γ-Cadinene | 14.06 | 38.33 | - | 4.48 | 3.14 | 4.52 | 111.99 | - | 27.02 | 6.25 | 14.45 | 12.19 | - | 17.90 | 3.06 | 2.2 | 0.29 | - | 7.99 | 0.13 |
| α-Cedrene | 1.94 | - | 1.04 | - | - | 1.21 | - | 1.19 | - | - | 3.36 | - | 11.11 | - | - | 2.3 | - | 13.7 | - | - |
| Ledol | 17.41 | 58.69 | - | 121.81 | 0.67 | 3.34 | 11.41 | - | 33.22 | 3.79 | 10.29 | - | - | 20.04 | - | 7.48 | - | - | 2.87 | - |
| Guaiol | 18.78 | - | - | - | - | 6.47 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Terpinen-4-ol | 0.02 | 2.69 | 0.56 | 0.41 | 0.67 | 0.74 | 2.76 | 0.96 | 3.57 | 0.61 | 1.02 | 3.48 | 1.42 | 3.61 | 0.57 | 2.39 | 1.41 | 2.40 | 2.31 | 2.04 |
| α-Cubebene | 0.27 | - | - | - | - | 18.38 | - | - | - | - | 14.35 | - | - | - | - | 13.06 | - | - | - | - |
| β-Guaiene | 7.17 | 7.25 | 7.1 | 46.17 | 0.90 | 1.37 | 2.21 | 3.07 | 12.28 | 2.00 | 3.65 | - | 5.10 | 0.18 | 1.33 | 8.43 | - | 7.83 | - | 2.75 |
| α-Acorenol | - | 46.55 | - | 10.43 | 1.32 | - | 17.34 | - | 1.61 | 0.38 | - | 0.85 | - | 8.28 | - | - | - | - | 1.38 | - |
| Geranyl linallol | - | 27.96 | 12.08 | 3.05 | - | - | 3.27 | 4.62 | 0.41 | - | - | 10.05 | - | 3.40 | - | - | 3.32 | - | 0.05 | - |
| Cryptomeridiol | - | 490.83 | - | 325.26 | 6.07 | - | 451.33 | - | 35.87 | 2.33 | - | 72.54 | - | 187.50 | 118.3 | - | 41.57 | - | 97.54 | 99.32 |
| Farnesol | - | 64.70 | - | - | - | - | 418.58 | - | - | - | - | 90.61 | - | - | - | - | 6.36 | - | - | - |
| β-Germacrene | - | 2.14 | - | - | - | - | 24.78 | - | - | - | - | 23.86 | - | - | - | - | 8.93 | - | - | - |
| Drimenol | - | 10.99 | - | - | - | - | 167.76 | - | - | - | - | 35.62 | - | - | - | - | 8.99 | - | - | - |
| 2,4-Decadienal | - | 17.74 | 31.81 | - | - | - | - | 59.58 | - | - | - | - | 86.12 | - | - | - | - | 1.88 | - | - |
| Curcumol | - | 62.08 | 12.59 | 3.54 | 2.77 | - | 81.12 | 38.73 | 83.26 | 29.34 | - | 278.03 | 92.62 | 230.52 | 22.95 | - | 104.79 | 61.94 | 102.41 | 125.16 |
| Viridiflorol | - | 11.14 | - | - | - | - | 11.47 | - | - | - | - | 3.75 | - | - | - | - | 0.71 | - | - | - |
| α-Pinene | - | 28.60 | - | - | - | - | 14.38 | - | - | - | - | 6.16 | - | - | - | - | - | - | - | - |
| Hinokitiol | - | - | 3.53 | - | - | - | - | 1.09 | - | - | - | - | - | - | - | - | - | - | - | - |
| Geranylacetone | - | - | 5.10 | - | 22.59 | - | - | 1.63 | - | 11.40 | - | - | 3.83 | - | 22.70 | - | - | 2.26 | - | 76.44 |
| 7-epi-Sesquithujene | - | - | 11.20 | - | - | - | - | 0.97 | - | - | - | - | - | - | - | - | - | - | - | - |
| α-curcumene | - | - | 22.31 | - | - | - | - | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - |
| (E)-2-Heptenal | - | - | 16.51 | - | - | - | - | 28.22 | - | - | - | - | 37.35 | - | - | - | - | 15.61 | - | - |
| Citronellol | - | - | 0.74 | - | - | - | - | 0.61 | - | - | - | - | - | - | - | - | - | - | - | - |
| 2,6-Dimethyl-5-heptenal | - | - | 2.68 | - | - | - | - | 0.9 | - | - | - | - | - | - | - | - | - | - | - | - |
| (−)-Epiglobulol | - | - | 14.68 | 7.42 | - | - | - | 4.01 | 0.12 | - | - | - | - | 4.64 | - | - | - | - | 4.80 | - |
| β-Bisabolene | - | - | - | 7.96 | - | - | - | - | 0.62 | - | - | - | - | 4.26 | - | - | - | - | 2.66 | - |
| β-Sesquiphellandrene | - | - | - | 7.85 | - | - | - | - | 2.09 | - | - | - | - | 17.66 | - | - | - | - | 10.78 | - |
| β-Selinene | - | - | - | 10.41 | - | - | - | - | 10.69 | - | - | - | - | 38.47 | - | - | - | - | 32.34 | - |
| Aromandendrene | - | - | - | 0.04 | - | - | - | - | 0.11 | - | - | - | - | 14.86 | - | - | - | - | 0.03 | - |
| α-Terpieol | - | - | - | 0.69 | - | - | - | - | 8.89 | - | - | - | - | 35.63 | - | - | - | - | 24.61 | - |
| α-Cadinene | - | - | - | 2.5 | - | - | - | - | 19.90 | - | - | - | - | 34.11 | - | - | - | - | 17.30 | - |
| (E)-2-Octen-1-ol | - | - | - | - | 0.52 | - | - | - | - | 0.07 | - | - | - | - | - | - | - | - | - | - |
| (E)-2-Decenal | - | - | - | - | 0.79 | - | - | - | - | 0.27 | - | - | - | - | - | - | - | - | - | - |
| 7d a | 14d | 21d | 28d | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | SPS1 | SPQ1 | SPG1 | SPH1 | SPS1 | SPQ1 | SPG1 | SPH1 | SPS1 | SPQ1 | SPG1 | SPH1 | SPS1 | SPQ1 | SPG1 | SPH1 |
| DL-Menthol | 700.31 | 75.73 | 349.66 | 100.00 | 147.29 | 18.86 | 445.01 | 64.4 | 1438.62 | 113.04 | 768.60 | 20.94 | 836.15 | 51.86 | 110.49 | 69.62 |
| elemol | 77.06 | 66.33 | 25.75 | 30.91 | 29.34 | 8.71 | 662.22 | 20.37 | 13.46 | 51.85 | 147.04 | 21.71 | 0.66 | 36.26 | 31.18 | 70.26 |
| linalool | 53.35 | 28.33 | 19.58 | 228.06 | 13.51 | 11.02 | 1.70 | 196.35 | 89.38 | 15.78 | 6.31 | 152.85 | 18.67 | 12.96 | 2.53 | 150.71 |
| (+)-β-Funebrene | 67.94 | 16.35 | 7.91 | 13.37 | 34.01 | 12.81 | 0.94 | 2.81 | 1187.67 | - | - | 4.94 | 653.87 | - | - | 2.85 |
| Cedrenol | 4.84 | 4.26 | - b | 54.29 | 28.90 | 2.65 | - | 82.05 | 85.50 | 13.88 | - | 52.84 | 47.99 | 6.36 | - | 227.88 |
| Cedrene | 57.96 | 9.76 | 13.31 | 45.93 | 67.01 | 5.72 | 52.92 | 9.29 | 257.70 | 13.44 | 21.29 | - | 288.99 | 21.29 | 3.77 | - |
| 4(15),5,10(14)-Germacratrien-1-ol | 472.38 | 544.4 | 25.56 | 24.11 | 166.53 | 194.32 | 321.01 | 7.66 | 894.35 | 1086.38 | 505.23 | 5.29 | 449.34 | 207.60 | 110.84 | 43.69 |
| β-elemene | 20.62 | 78.64 | 9.20 | 94.04 | 4.15 | 39.55 | 101.27 | 103.06 | 111.34 | 93.38 | 227.40 | 49.55 | 47.69 | 21.49 | 12.38 | 23.08 |
| nerolidol | 251.76 | 257.74 | 125.31 | 11.16 | 38.50 | 37.51 | 174.53 | 0.35 | 299.02 | 36.79 | 69.46 | 4.91 | 203.46 | 45.58 | 5.41 | 2.76 |
| fitone | 271.44 | 185.17 | 75.90 | 132.75 | 96.78 | 101.57 | 107.48 | 57.34 | 680.14 | 539.82 | 90.11 | 64.83 | 597.40 | 280.87 | 22.89 | 62.54 |
| (+)-β-cedrene | 820.81 | 511.88 | - | - | 403.67 | 174.23 | - | - | 8686.98 | 910.26 | - | - | 4676.51 | 546.06 | - | - |
| β-eudesmol | 7012.8 | 7198.94 | 533.63 | 1182.41 | 2787.03 | 2660.94 | 3846.91 | 929.44 | 12288.20 | 6246.61 | 1649.91 | 1525.05 | 7560.39 | 2844.84 | 709.76 | 613.44 |
| Phytol | - | 3.89 | 1.58 | - | - | 3.94 | 0.84 | - | - | 5.81 | 1.61 | - | - | 6.55 | 0.92 | - |
| (E)-2-Octenal | 7.80 | 10.11 | 150.01 | 0.94 | 3.39 | 4.63 | 142.28 | 1.38 | 12.23 | 4.67 | 71.44 | 1.93 | 5.14 | 2.95 | 33.19 | 3.30 |
| Geranyl isovalerate | 11.52 | 16.51 | 1.05 | 6.77 | 3.67 | 5.75 | 0.50 | 2.77 | 2.90 | 7.38 | - | 0.14 | 0.17 | 5.19 | - | - |
| Nerol | 23.41 | 4.71 | - | - | 3.53 | 0.81 | - | - | 3.67 | 9.81 | - | - | 0.96 | 14.97 | - | - |
| α-bulnesene | 211.59 | 226.06 | 6.60 | 251.36 | 43.97 | 65.28 | 249.92 | 189.03 | 180.47 | 141.61 | 6.93 | 111.42 | 89.61 | 94.31 | - | 91.05 |
| (−)-Globulol | - | 11.48 | - | - | - | 3.98 | - | - | - | 48.57 | - | - | - | 62.98 | - | - |
| α-Agarofuran | - | - | 1.11 | - | - | - | 0.33 | - | - | - | - | - | - | - | - | - |
| δ-Cadinene | - | - | 2.90 | 161.88 | - | - | 87.46 | 143.22 | - | - | 94.52 | 76.83 | - | - | 5.49 | 48.86 |
| 2-Undecenal | - | - | 63.73 | - | - | - | 22.48 | - | - | - | 35.12 | - | - | - | 8.37 | - |
| β-Caryophyllene | - | - | 2.45 | - | - | - | 54.60 | - | - | - | 36.14 | - | - | - | 1.79 | - |
| (−)-Humulene epoxide II | - | - | 5.84 | - | - | - | 107.95 | - | - | - | 109.68 | - | - | - | 5.97 | - |
| Farnesal | - | - | 207.59 | - | - | - | 1358.24 | - | - | - | 256.49 | - | - | - | 4.00 | - |
| α-Farnesene | - | - | 17.32 | - | - | - | 162.85 | - | - | - | 143.13 | - | - | - | 6.79 | - |
| Cembrene | - | - | 95.03 | - | - | - | 328.25 | - | - | - | 86.54 | - | - | - | 39.11 | - |
| (E)-β-Famesene | - | - | 48.83 | - | - | - | 276.67 | - | - | - | 72.34 | - | - | - | 4.64 | - |
| α-Bisabolol | 17.42 | 10.74 | 366.59 | - | 19.75 | 4.6 | 160.73 | - | 17.40 | 2.39 | 550.95 | - | 46.14 | 12.84 | 281.55 | - |
| 1-Octen-3-ol | - | 1.77 | 663.52 | - | - | 0.47 | 3019.5 | - | - | - | 231.02 | - | - | - | 42.56 | - |
| trans-Farnesol | - | - | 427.86 | - | - | - | 2873.61 | - | - | - | 934.86 | - | - | - | 68.99 | - |
| α-Gurjenene | - | - | - | 32.33 | - | - | - | 5.88 | - | - | - | 0.24 | - | - | - | - |
| γ-Cadinene | - | 1.83 | 0.66 | 57.52 | - | 1.17 | 88.52 | 60.09 | - | - | 9.36 | 38.24 | - | - | 0.29 | 10.32 |
| α-Cedrene | 1.04 | - | - | 1.94 | 1.19 | - | - | 1.21 | 11.11 | - | - | 3.36 | 13.7 | - | - | 2.30 |
| Ledol | - | 121.73 | - | 76.85 | - | 13.10 | - | 38.66 | - | 8.32 | - | 22.01 | - | 2.87 | - | 7.48 |
| Guaiol | - | - | - | 18.78 | - | - | - | 6.47 | - | - | - | - | - | - | - | - |
| Terpinen-4-ol | - | - | - | 4.35 | - | - | - | 8.64 | - | - | - | 10.1 | - | -- | - | 10.55 |
| α-Cubebene | - | - | - | 0.27 | - | - | - | 18.38 | - | - | - | 14.35 | - | - | - | 13.06 |
| β-Guaiene | - | 53.42 | - | 15.17 | - | 14.49 | - | 6.44 | - | 0.18 | - | 10.08 | - | - | - | 19.01 |
| α-Acorenol | 27.22 | 29.92 | 1.16 | - | 2.34 | 9.15 | 7.84 | - | - | 8.28 | 0.85 | - | - | 1.38 | - | - |
| Geranyl linallol | 7.40 | 35.69 | - | - | 3.04 | 5.26 | - | - | 3.40 | 10.05 | - | - | 0.05 | 3.32 | - | - |
| Cryptomeridiol | 63.00 | 617.28 | 10.59 | 131.29 | 0.67 | 80.31 | 394.59 | 13.96 | - | 39.28 | 337.72 | 1.34 | - | 35.64 | 202.79 | - |
| Farnesol | - | - | 64.70 | - | - | - | 418.58 | - | - | - | 90.61 | - | - | - | 6.36 | - |
| β-Germacrene | - | - | 2.14 | - | - | - | 24.78 | - | - | - | 23.86 | - | - | - | 8.93 | - |
| Drimenol | - | - | 10.99 | - | - | - | 167.76 | - | - | - | 35.62 | - | - | - | 8.99 | - |
| 2,4-Decadienal | - | - | 49.55 | - | - | - | 59.58 | - | - | - | 86.12 | - | - | - | 1.88 | - |
| Curcumol | - | - | - | 80.98 | - | - | - | 232.45 | - | - | - | 624.12 | - | - | - | 394.30 |
| Viridiflorol | - | - | - | 11.14 | - | - | - | 11.47 | - | - | - | 3.75 | - | -- | - | 0.71 |
| α-Pinene | - | - | - | 28.60 | - | - | - | 14.38 | - | - | - | 6.16 | - | - | - | - |
| Hinokitiol | - | 3.53 | - | - | - | 1.09 | - | - | - | - | - | - | - | - | - | - |
| Geranylacetone | 15.90 | 3.35 | 6.82 | 1.62 | 7.10 | 3.30 | 1.91 | 0.72 | 11.79 | 4.12 | 10.01 | 0.61 | 55.41 | 19.49 | 2.57 | 1.23 |
| 7-epi-sesquithujene | - | - | 11.2 | - | - | - | 0.97 | - | - | - | - | - | - | - | - | - |
| α-curcumene | - | - | 22.31 | - | - | - | 0.02 | - | - | - | - | - | - | - | - | - |
| (E)-2-Heptenal | - | - | 16.51 | - | - | - | 28.22 | - | - | - | 37.35 | - | - | - | 15.61 | - |
| Citronellol | - | - | 0.74 | - | - | - | 0.61 | - | - | - | - | - | - | - | - | - |
| 2,6-Dimethyl-5-heptenal | - | - | 2.68 | - | - | - | 0.90 | - | - | - | - | - | - | - | - | - |
| (−)-Epiglobulol | - | - | 7.42 | 14.68 | - | - | 0.12 | 4.01 | - | - | 4.64 | - | - | - | 4.80 | - |
| β-Bisabolene | 7.96 | - | - | - | 0.62 | - | - | - | 4.26 | - | - | - | 2.66 | - | - | - |
| β-Sesquiphellandrene | 7.85 | - | - | - | 2.09 | - | - | - | 17.66 | - | - | - | 10.78 | - | - | - |
| β-selinene | - | - | 8.40 | 2.01 | - | - | 0.64 | 10.05 | - | - | 21.72 | 16.75 | - | - | 17.38 | 14.96 |
| Aromandendrene | - | - | 0.04 | - | - | - | 0.11 | - | - | - | 14.86 | - | - | - | 0.03 | - |
| α-Terpieol | - | - | - | 0.69 | - | - | - | 8.89 | - | - | - | 35.63 | - | - | - | 24.61 |
| α-Cadinene | - | - | - | 2.50 | - | - | - | 19.90 | - | - | - | 34.11 | - | - | - | 17.3 |
| (E)-2-Octen-1-ol | - | 0.52 | - | - | - | 0.07 | - | - | - | - | - | - | - | - | - | - |
| (E)-2-Decenal | - | 0.79 | - | - | - | 0.27 | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, M.; Huo, Q.; Gan, L.; Chen, Y.; Xiao, D.; Guo, X. Effects of Four Strains of Actinomycetes on the Content of Terpenoids in Baijiu. Foods 2023, 12, 1494. https://doi.org/10.3390/foods12071494
Feng M, Huo Q, Gan L, Chen Y, Xiao D, Guo X. Effects of Four Strains of Actinomycetes on the Content of Terpenoids in Baijiu. Foods. 2023; 12(7):1494. https://doi.org/10.3390/foods12071494
Chicago/Turabian StyleFeng, Minxue, Qiaojuan Huo, Linyao Gan, Yefu Chen, Dongguang Xiao, and Xuewu Guo. 2023. "Effects of Four Strains of Actinomycetes on the Content of Terpenoids in Baijiu" Foods 12, no. 7: 1494. https://doi.org/10.3390/foods12071494