Plant-Based Alternatives to Cheese Formulated Using Blends of Zein and Chickpea Protein Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients
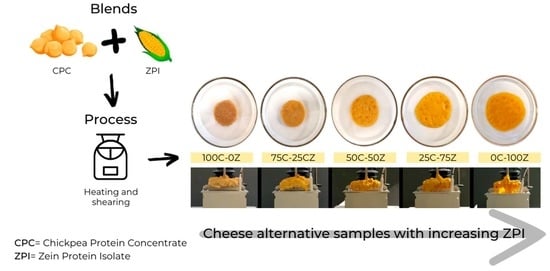
2.2. Formulation of Cheese Alternative Samples
2.3. Compositional and Colour Analyses of Powder Ingredients and Cheese Alternatives
2.4. Protein Profile Analysis of Protein Ingredients
2.5. Differential Scanning Calorimetry Analysis of the Ingredients and Cheese Alternatives
2.6. Confocal Laser Scanning Microscopy
2.7. Dynamic Low Amplitude Oscillatory Shear Rheology
2.8. Schreiber Meltability Test
2.9. Extensibility Analysis
2.10. Texture Profile Analysis
2.11. Statistical Data Analysis
3. Results and Discussion
3.1. Composition of Powder Ingredients and Cheese Alternatives
3.1.1. Chemical Composition
3.1.2. Colour
3.1.3. Protein Profile of Powder Ingredients
3.2. Thermal Behaviour of the Powder Ingredients and Cheese Alternatives
3.3. Microstructure
3.4. Rheological Properties
3.5. Meltability
3.6. Stretchability
3.7. Textural Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McSweeney, P.L.; Ottogalli, G.; Fox, P.F. Diversity of Cheese Varieties: An Overview. In Cheese: Chemistry, Physics and Microbiology. Volume 2: Major Cheese Groups; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Ltd.: San Diego, CA, USA, 2004; Volume 2, pp. 1–22. [Google Scholar]
- Clay, N.; Garnett, T.; Lorimer, J. Dairy intensification: Drivers, impacts and alternatives. Ambio 2020, 49, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tso, R.; Lim, A.J.; Forde, C.G.; Sg, A.J.L. A Critical Appraisal of the Evidence Supporting Consumer Motivations for Alternative Proteins. Foods 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2020, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; McClements, D.J. The science of plant-based foods: Approaches to create nutritious and sustainable plant-based cheese analogs. Trends Food Sci. Technol. 2021, 118, 207–229. [Google Scholar] [CrossRef]
- Good Food Institute and SPINS. U.S. Retail Market Insights: Plant-Based Foods. 2021. Available online: https://gfi.org/wp-content/uploads/2022/03/2021-U.S.-retail-market-insights_Plant-based-foods-GFI.pdf (accessed on 5 April 2022).
- Grasso, N.; Roos, Y.H.; Crowley, S.V.; Arendt, E.K.; O’Mahony, J.A. Composition and physicochemical properties of commercial plant-based block-style products as alternatives to cheese. Futur. Foods 2021, 4, 100048. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Mefleh, M.; Pasqualone, A.; Caponio, F.; Faccia, M. Legumes as basic ingredients in the production of dairy-free cheese alternatives: A review. J. Sci. Food Agric. 2021, 102, 8–18. [Google Scholar] [CrossRef]
- Hall, C.; Hillen, C.; Robinson, J.G. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, N. Characterization of protein isolates from different Indian chickpea (Cicer arietinum L.) cultivars. Food Chem. 2007, 102, 366–374. [Google Scholar] [CrossRef]
- Papalamprou, E.M.; Doxastakis, G.I.; Biliaderis, C.G.; Kiosseoglou, V. Influence of preparation methods on physicochemical and gelation properties of chickpea protein isolates. Food Hydrocoll. 2009, 23, 337–343. [Google Scholar] [CrossRef]
- Withana-Gamage, T.S.; Wanasundara, J.P.; Pietrasik, Z.; Shand, P.J. Physicochemical, thermal and functional characterisation of protein isolates from Kabuli and Desi chickpea (Cicer arietinum L.): A comparative study with soy (Glycine max) and pea (Pisum sativum L.). J. Sci. Food Agric. 2011, 91, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Grasso, N.; Bot, F.; Roos, Y.H.; Crowley, S.V.; Arendt, E.K.; Mahony, J.A.O. The influence of protein concentration on key quality attributes of chickpea-based alternatives to cheese. Curr. Res. Food Sci. 2022, 5, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Mattice, K.D.; Marangoni, A.G. Physical properties of plant-based cheese products produced with zein. Food Hydrocoll. 2020, 105, 105746. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Anderson, T.J.; Lamsa, B.P. Zein extraction from corn, corn products, and coproducts and modifications for various applications: A review. Cereal Chem. 2011, 88, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Argos, P.; Pedernes, K.; Marks, M.D.; Larkins, B.A. A structural model for maize zein proteins. J. Biol. Chem. 1982, 257, 9984–9990. [Google Scholar] [CrossRef]
- Smith, B.M.; Bean, S.R.; Selling, G.; Sessa, D.; Aramouni, F.M. Role of non-covalent interactions in the production of visco-elastic material from zein. Food Chem. 2014, 147, 230–238. [Google Scholar] [CrossRef]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef] [Green Version]
- Mattice, K.D.; Marangoni, A.G. Evaluating the Use of Zein in Structuring Plant-Based Products. Curr. Res. Food Sci. 2020, 3, 59–66. [Google Scholar] [CrossRef]
- Jiménez-Munoz, L.M.; Tavares, G.M.; Corredig, M. Design future foods using plant protein blends for best nutritional and technological functionality. Trends Food Sci. Technol. 2021, 113, 139–150. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Soto-Reyes, N.; Dávila-Rodríguez, M.; Lorenzo-Leal, A.C.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Plant-Based Milk Alternatives: Types, Processes, Benefits, and Characteristics. Food Rev. Int. 2021, 00, 1–32. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis-Protein in Dried Milk 930.29 1930.
- AOAC Official Methods of Analysis-Determination of Nitrogen (Total) in Cheese 2001.14 2002.
- AOAC Official Methods of Analysis-Solids (total) and loss on drying (moisture) in flour. Air oven method 925.10 1925.
- AOAC Official Methods of Analysis-Moisture in Cheese 926.08 1990.
- AOAC Official Methods of Analysis-Ash of flour 923.03 1923.
- AOAC Official Methods of Analysis-Ash of Cheese 935.42 1990.
- AACC Approved Methods of Analysis-Crude Fat in Wheat, Corn, and Soy Flour, Feeds, and Mixed Feeds 30-25.01 2009.
- Le Tohic, C.; O’Sullivan, J.J.; Drapala, K.P.; Chartrin, V.; Chan, T.; Morrison, A.P.; Kerry, J.P.; Kelly, A.L. Effect of 3D printing on the structure and textural properties of processed cheese. J. Food Eng. 2018, 220, 56–64. [Google Scholar] [CrossRef]
- Auty, M.A.E.; Twomey, M.; Guinee, T.P.; Mulvihill, D.M. Development and application of confocal scanning laser microscopy methods for studying the distribution of fat and protein in selected dairy products. J. Dairy Res. 2001, 68, 417–427. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Cheese structure, rheology and texture. In Fundamentals of Cheese Science; Fox, P.F., Guinee, T.P., Cogan, T.M., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2017; pp. 475–532. ISBN 9781489976819. [Google Scholar]
- Altan, A.; Turhan, M.; Gunasekaran, S. Short communication: Comparison of covered and uncovered Schreiber test for cheese meltability evaluation. J. Dairy Sci. 2005, 88, 857–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramel, P.R.; Marangoni, A.G. Processed cheese as a polymer matrix composite: A particle toolkit for the replacement of milk fat with canola oil in processed cheese. Food Res. Int. 2018, 107, 110–118. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Gulewicz, P.; Frias, J.; Gulewicz, K.; Vidal-Valverde, C. Assessment of protein fractions of three cultivars of Pisum sativum L.: Effect of germination. Eur. Food Res. Technol. 2008, 226, 1465–1478. [Google Scholar] [CrossRef]
- Wallace, J.C.; Lopes, M.A.; Paiva, E.; Larkins, B.A. New Methods for Extraction and Quantitation of Zeins Reveal a High Content of γ-Zein in Modified opaque-2 Maize1. Plant Physiol 1990, 92, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Hamaker, B.R.; Mohamed, A.A.; Habben, J.E.; Huang, C.P.; Larkins, B.A. Efficient procedure for extracting maize and sorghum kernel proteins reveals higher prolamin contents than the conventional method. Cereal Chem. 1995, 72, 583–588. [Google Scholar]
- Esen, A. A proposed nomenclature for the alcohol-soluble proteins (zeins) of maize (Zea mays L.). J. Cereal Sci. 1987, 5, 117–128. [Google Scholar] [CrossRef]
- Wilson, C.M. Multiple Zeins from Maize Endosperms Characterized by Reversed-Phase High Performance Liquid Chromatography. Plant Physiol 1991, 95, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Day, L. Proteins from land plants-Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Breuninger, W.F.; Piyachomkwan, K.; Sriroth, K. Tapioca/Cassava Starch: Production and Use. In Starch; BeMiller, J., Whistler, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 541–568. ISBN 9780127462752. [Google Scholar]
- Zheng, M.; Xiao, Y.; Yang, S.; Liu, M.; Feng, L.; Ren, Y.; Yang, X.; Lin, N.; Liu, J. Effect of adding zein, soy protein isolate and whey protein isolate on the physicochemical and in vitro digestion of proso millet starch. Int. J. Food Sci. Technol. 2020, 55, 776–784. [Google Scholar] [CrossRef]
- Kett, A.P.; Bruen, C.M.; O’halloran, F.; Chaurin, V.; Lawlor, P.G.; O’mahony, J.A.; Giblin, L.; Fenelon, M.A. The effect of a-or b-casein addition to waxy maize starch on postprandial levels of glucose, insulin, and incretin hormones in pigs as a model for humans. Food Nutr. Res. 2012, 56, 7989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeka, H.; Kokinii, J.L. Effect of Glass Transition and Cross-Linking on Rheological Properties of Zein: Development of a Preliminary State Diagram. Cereal Chem. 1996, 73, 433–438. [Google Scholar]
- Dai, L.; Sun, C.; Wei, Y.; Mao, L.; Gao, Y. Characterization of Pickering emulsion gels stabilized by zein/gum arabic complex colloidal nanoparticles. Food Hydrocoll. 2018, 74, 239–248. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Salgado, A.M.; Holding, D.R.; Campanella, O.H.; Hamaker, B.R. Dispersion of zein into pea protein with alkaline agents imparts cohesive and viscoelastic properties for plant-based food analogues. Food Hydrocoll. 2023, 134, 108044. [Google Scholar] [CrossRef]
- Muthukumarappan, K.; Wang, Y.C.; Gunasekaran, S. Short Communication: Modified Schreiber Test for Evaluation of Mozzarella Cheese Meltability. J. Dairy Sci. 1999, 82, 1068–1071. [Google Scholar] [CrossRef]
- Lucey, J.A.; Johnson, M.E.; Horne, D.S. Invited Review: Perspectives on the Basis of the Rheology and Texture Properties of Cheese. J. Dairy Sci. 2003, 86, 2725–2743. [Google Scholar] [CrossRef] [Green Version]
- Bourne, M.C. Principles of Objective Texture Measurements. In Food Texture and Viscosity; Bourne, M.C., Ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 107–188. ISBN 9780121190620. [Google Scholar]
- Cunha, C.R.; Dias, A.I.; Viotto, W.H. Microstructure, texture, colour and sensory evaluation of a spreadable processed cheese analogue made with vegetable fat. Food Res. Int. 2010, 43, 723–729. [Google Scholar] [CrossRef]









| ZPI | CPC | Starch | Shea Butter | Lecithin | Lactic Acid | Water | |
|---|---|---|---|---|---|---|---|
| 0Z-100C | 0 | 29.9 | 0.10 | 14.4 | 0.20 | 8.50 | 48.2 |
| 25Z-75C | 4.90 | 22.5 | 2.80 | 14.1 | 0.20 | 6.30 | 50.4 |
| 50Z-50C | 9.80 | 15.0 | 5.40 | 13.8 | 0.20 | 4.30 | 52.4 |
| 75Z-25C | 14.6 | 7.50 | 8.00 | 13.6 | 0.20 | 2.10 | 54.6 |
| 100Z-0C | 19.5 | 0 | 10.6 | 13.3 | 0.20 | 0.10 | 56.6 |
| Protein (%) | Moisture (%) | Carbohydrate (%) | Fat (%) | Ash (%) | pH (-) | aw (-) | |
|---|---|---|---|---|---|---|---|
| 0Z-100C | 16.3 ± 0.26 a | 56.2 ± 0.01 a | 11.1 | 15.3 ± 0.33 ab | 1.40 ± 0.04 a | 4.55 ± 0.01 a | 0.985 ± 0.000 e |
| 25Z-75C | 16.2 ± 0.17 a | 56.8 ± 0.52 a | 10.9 | 15.0 ± 0.13 b | 1.01 ± 0.01 b | 4.54 ± 0.02 a | 0.988 ± 0.001 d |
| 50Z-50C | 16.1 ± 0.06 a | 56.7 ± 0.04 a | 11.4 | 15.0 ± 0.37 b | 0.73 ± 0.03 c | 4.55 ± 0.01 a | 0.992 ± 0.002 c |
| 75Z-25C | 16.3 ± 0.17 a | 56.3 ± 0.20 a | 11.6 | 15.5 ± 0.11 ab | 0.49 ± 0.02 d | 4.53 ± 0.02 a | 0.994 ± 0.001 b |
| 100Z-0C | 16.3 ± 0.30 a | 56.2 ± 0.34 a | 10.7 | 15.9 ± 0.34 a | 0.22 ± 0.01 e | 4.53 ± 0.01 a | 0.997 ± 0.001 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grasso, N.; Bot, F.; Roos, Y.H.; Crowley, S.V.; Arendt, E.K.; O’Mahony, J.A. Plant-Based Alternatives to Cheese Formulated Using Blends of Zein and Chickpea Protein Ingredients. Foods 2023, 12, 1492. https://doi.org/10.3390/foods12071492
Grasso N, Bot F, Roos YH, Crowley SV, Arendt EK, O’Mahony JA. Plant-Based Alternatives to Cheese Formulated Using Blends of Zein and Chickpea Protein Ingredients. Foods. 2023; 12(7):1492. https://doi.org/10.3390/foods12071492
Chicago/Turabian StyleGrasso, Nadia, Francesca Bot, Yrjo H. Roos, Shane V. Crowley, Elke K. Arendt, and James A. O’Mahony. 2023. "Plant-Based Alternatives to Cheese Formulated Using Blends of Zein and Chickpea Protein Ingredients" Foods 12, no. 7: 1492. https://doi.org/10.3390/foods12071492